A New Heuristic for Lifestyle Strategy
In April of 2015 I was privileged to be able to present at the Institute for Human Machine Cognition in Pensacola, Florida. For those of you not familiar with the work of IHMC, they are a not-for-profit research institute focused on artificial intelligence, robotics, and related areas. In addition to their work in robotics, however, they also host a regular seminar series for the community.
My approximately 1-hour-long presentation starts with an idea: that there exists a “heuristic”, a mental strategy or shortcut, through which lifestyle decisions can be passed in order to achieve optimal health. The video goes into more details and I recommend watching it.
If there can be said to be such a thing, it is this…
What choices can I make that will result in my body becoming more resilient to stress?
This is because all of the primary causes of aging have stress at their root. However, somewhat paradoxically, stress isn’t always bad. Short-term stress can result in a reduction in long-term, chronic stress. In other words, we can build resilience. Exercise works this way, and so does even drinking green tea. In fact, many beneficial compounds work by inducing a type of “hormesis”, which causes your body to up-regulate its own built-in antioxidant and repair systems.
Tricking our body with hormetic substances like green tea isn’t our only option for building resilience to stress. Stress is a consequence of being alive. Even as you read this, the mitochondria that generate the energy for your cells to do their work are producing oxidative damage as a byproduct, which must be sequestered…and for what slips through the cracks, repairs must be made.
“I think the low hanging fruit in getting us all to live long lives is in nutrition.” - Dr. Bruce Ames
Dr. Bruce Ames triage theory posits that when faced with micronutrient inadequacy, our body will always choose distribute the micronutrients we’ve got to optimize for short-term survival and reproduction at the cost of allowing insidious damage to slip through the floodgates and endanger our long-term health. Maybe this is cholesterol build up in our arteries in the form of atherosclerotic plaques, or maybe it’s shortening of our telomeres leading to eventual cellular senescence and death.
It isn’t sufficient, however, to simply get a lot of micronutrients. The important thing is to get the right amount of nutrients for our genetic background. Whether we’re talking about the distribution of minerals like selenium in our soils, or even the actual whole food make-up of our diet, there is one thing we can be certain of… our collective ancestral past is filled with great adaptive diversity, and pinning down what our genes have left us best suited for is what will ultimately allow us to find our own biological optimal point that keeps us from straying too far off the mark from “optimal sufficiency” into toxicity, or insufficiency.
Empowering individuals to find their own biological “sweet spot” is probably humanity’s best hope for increasing lifespan, next to something more drastic, like genetic engineering, in the next few decades. Thankfully, with the advent of tools like 23andMe and Promethease, as well as the emergence of consumer blood testing companies, individuals are more empowered now than they ever have. Especially if they know what genes to look at.
Nutrigenomics
Hundreds of biochemical pathways require micronutrients, which are ~30-40 essential vitamins, minerals, amino acids, and fatty acids, that we must get from our diet. In fact, approximately 22% of the genes that encode for enzymes (which make energy, make antioxidants, repair damage etc.) require micronutrients as cofactors, which means they need them to function properly. Without these micronutrients, these enzymes dysfunction and can lead to acute disease as well as diseases of aging. In order to prevent acute disease, daily recommended intakes (DRI) of these micronutrients have been set to ensure we get adequate amounts of them. However, we know that we are not meeting these RDAs.
One of the main reasons for such widespread micronutrient inadequacies is that people are not eating micronutrient dense foods, including a wide variety of vegetables. In addition, the dietary requirements for many micronutrients are based on the normal population, but we are all different. There are single nucleotide polymorphisms (SNPs), which refer to just one change in nucleotide in your DNA, and this change may alter the function of the gene. Gene polymorphisms are different from mutations, which occur randomly on an individual basis, because polymorphisms are represented in some significant portion of the population (greater than 1% of the population), and are presumed to have been "selected for for some reason.” Polymorphisms affect phenotype (such as hair and eye color), affect our disease risk (such as Alzheimer’s disease and prostate cancer), and also influence the way we absorb and metabolize micronutrients. The effect of gene polymorphisms on micronutrient absorption and metabolism is known as nutrigenomics and I have discussed this topic more in depth in a previous video.
There are a couple of common polymorphisms in genes that metabolize vitamin D3. Vitamin D3 gets metabolized to 25-hydroxyvitamin D in the liver by an enzyme called CYP2R1. The 25-hydroxyvitamin D is then converted into the active steroid hormone (1,25-hydroxyvitamin D) in the kidneys. 25-hydroxyvitamin D is the stable, circulating form of vitamin D that is measured as a proxy of vitamin D status. Levels below 20 ng/ml is considered deficient, less than 30 ng/ml is inadequate and between 30-60 ng/ml is considered adequate. Meta-analyses have shown that people with serum levels between 40-60ng/ml have the lowest all-cause mortality. Supplementation with vitamin D is a good way to ensure you get adequate vitamin D. It is known that supplementing with 1,000 IU of vitamin D3 per day generally raises serum 25-hydroxy levels by 5 ng/ml. This may not be the case for people with CYP2R1 polymorphisms as it has been shown that they cannot raise their serum vitamin D levels to the same degree as someone without this polymorphism with the dose of vitamin D.
In addition to gene polymorphisms, there are other factors that regulate our vitamin D levels. Since the primary source of vitamin D is UVB radiation from the sun, anything that blocks UVB rays also block the ability of our skin to produce vitamin D including sunscreen, melanin, living in a Northern latitude. A high percentage of body fat lowers the bioavailability of vitamin D.
You can learn more about vitamin D supplementation and answer popular vitamin D-related questions here.
Vitamin D-Related Polymorphisms
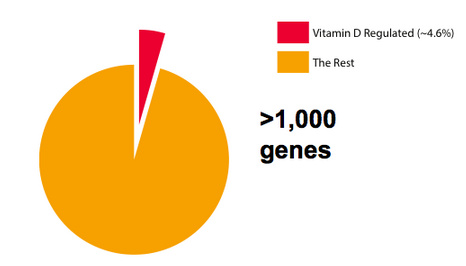
Vitamin D gets converted into a steroid hormone that controls the expression of over 1,000 genes in the human body. If you want to think of all the protein-encoding genes in the body as a piece of pie then vitamin D controls roughly 5% of those genes (Figure 1). There are many important functions in the body that are regulated by vitamin D, including processes that affect aging and brain function.
Folate-Related Polymorphisms
Folate is found in dark green leafy vegetables like spinach and it serves two very important functions in the body. First, folate serves as a precursor to make new DNA. This is important for repairing a damed cell and for producing new cells whether we are talking about a sperm cell, or a brain cell (Figure 2). Second, folate serves as a precursor to make epigenetic factors, which change how much of a gene a given cell produces so that the gene does either more or less of a certain function. Epigenetics is discussed in more detail below.
There are a cluster of common polymorphisms in the folate metabolism pathway that affect the way the body metabolizes folate. These polymorphisms are in a gene known as MTHFR and can decrease its function between 40-90% (Figure 2). I have discussed MTHFR polymorphisms in a previous video. Supplementation with L-methylfolate along with other B vitamins has been shown to help overcome the MTHFR deficit.
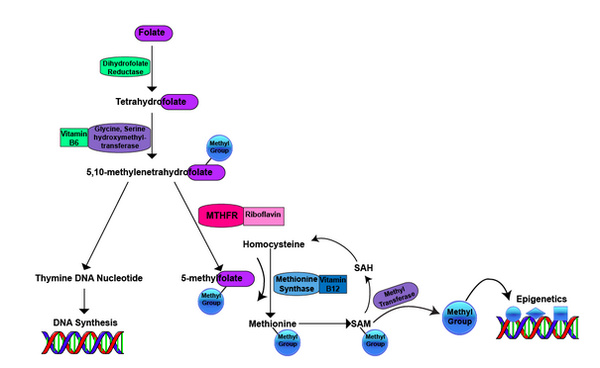
Figure 2: Folate Metabolism Pathway
Folate is converted into tetrahydrofolate, which is converted into 5,10-methylenetetrahydrofolate is required for two important pathways: 1). The synthesis of nucleic acids (thymine) required to make new DNA. 2). The production of 5-methylfolate by the riboflavin-dependent methylenetetrahydrofolate reductase (MTHFR) enzyme. The methyl group from 5-methylfolate is used to convert homocysteine into the essential amino acid methionine (via methylation) by the vitamin B12-dependent methionine synthase enzyme. Methionine, is now methylated and is used as methyl donor via methyl transferase enzymes for a broad range of methylation reactions involved in epigenetics (DNA methylation). Once the methyl group is used from SAM, the methionine forms homocysteine again and the cycle starts over.*
Epigenetics
Epigenetics refers to changes in the expression of genes but does not actually alter the genetic code itself. These epigenetic marks (like methyl groups from folate metabolism and acetyl groups) sit on top of your DNA and turn genes on and turn genes off. When a gene is turned on it is active, it is performing a certain function and the gene is often referred to as being expressed. When a gene is turned off it is not active, it is not performing a certain function so it is almost as if the gene is not there. When a gene is turned off it is often referred to as not being expressed. These epigenetic factors are regulated by our environment. For example, the type of food we eat, how much we eat, micronutrient intake, the time of day, how much sleep you get, stress, exercise, and heat all regulate changes in epigenetic factors and, thus, gene expression.
In addition, these epigenetic marks can be passed on to your children and grandchildren through the germline (ie. egg and sperm cells). For example, researchers have shown that when a male mouse is fed a bad diet, this not only affects him but also his offspring. Male mice that were fed an inflammatory diet consisting of corn oil starting when they were young, became obese and insulin resistant. Big surprise. What is even more surprising is that these obese male mice had female offspring that were fed a normal diet (not high in corn oil), did not become obese but did develop type 1 diabetes in adulthood. The high inflammatory diet silenced the gene in sperm DNA that encodes for protein that produces insulin in pancreatic beta islet cells and this was passed on to the female offspring resulting in less insulin production and type 1 diabetes.
Aging Epigenetic Signature
Another fascinating aspect about these epigenetic marks is that that follow a certain pattern with age. Blood samples taken from individuals ranging from 19 years old to 101 years old were analyzed and the methylation patterns were analyzed. Based on the methylation patterns of the blood cells researchers were able to identify the age of the individual with 96% accuracy within about 4 years. This suggest that there must be chronic signals that occur with age and these signals change epigenetic marks with age. These epigenetic factors, particularly methylation, change near certain genes involved in DNA repair, which repair DNA damage and prevent it from causing mutations that may lead to cancer and accelerated aging. Epigenetic factors also change near genes involved in stem cell function. Stem cells replenish cells in different organs so that it can continue to function. In addition, epigenetic factors also change near genes involved in metabolism, antioxidant function, stress resistance, and genes involved in age-related diseases (such as Alzheimer’s disease and cancer).
The question is: Is there anything we can do to delay or reverse these epigenetic changes that occur with age?
Limiting food has been shown to extend the lifespan of yeast, worms, flies, dogs, and monkeys suggesting this is an evolutionarily conserved mechanism. There are two paradigms: one involves eating ~30% less food than you would normally eat (which is called caloric restriction) and the other involves alternate day fasting (which is called intermittent fasting). Many studies have consistently found that decreasing food intake has profound changes on genes expression and increase the expression of genes involved in dealing with stress, increases the resilience to stress including the repair of damaged DNA, proteins and cells. All the same genes that decrease with age and have been shown to be epigenetically regulated with age are increased with caloric restriction and intermittent fasting.
There are similar benefits that are observed with both caloric restriction and intermittent fasting. Even just a few fours of intermittent fasting can cause epigenetic switches to turn on.
Sirt1: A Major Epigenetic Regulator
We know that being in a fasted state acts as an on switch for a gene called Sirt1, which is a global epigenetic regulator. Even a fasted state between meals is enough to act as an on switch for Sirt1.
Sirt1 is a link between nutrients, epigenetics, and longevity. Anytime we are in a “fed state” we produce energy from glucose from carbohydrates or or fatty acids from fat. But it requires energy and cofactors to produce energy from the foods we eat. A cofactor called nicotinamide adenine dinucleotide (or NAD, which can be made from niacin) is used in hundreds of metabolic reactions in the body. Once the NAD is used up, this generates NADH, which is an OFF switch for Sirt1. This balance is shifted in the fasting state: during the fasting state, there is more NAD around because it is not being used up and this serves as an ON switch for sirt1.
Even just a few hours of intermittent fasting can switch Sirt1 ON.
The mechanism by which Sirt1 regulates epigenetics is by removing epigenetic marks (known as acetyl groups) from histones, which is a DNA-protein complex that the DNA inside every cell is wound up in (Figure 2). This is how sirt1 changes genes expression of hundreds of genes including genes that make more mitochondria (Pgc1-alpha), genes that regulate circadian rhythm (clock genes), and it enhances the so-called longevity gene (Foxo3).
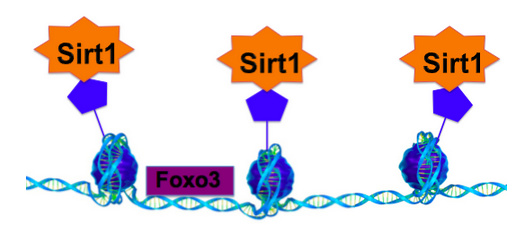
Figure 2: Sirt1 is a Global Epigenetic Regulator
Sirt1 removes epigenetic factors known as acetyl groups from histones, which are DNA protein complexes inside the cell. This results in a global change in gene expression such that many genes are turned on or off.
Stress Tolerance
I have previously discussed how the Foxo3 gene is associated with longevity in another video.
FOXO3 is a master regulator of many different genes involved in stress tolerance because it increases the production of genes that combat many of the types of damage that occur with age such as damage to DNA, proteins, and lipids, loss of stem cell function, and damaged cells (senescent). FOXO3 increases the production of genes including, DNA repair, tumor suppressor, anti-inflammatory, antioxidant, stem cell function, immune function, protein aggregation, and more. All the same genes that epigenetically decrease with age are all increased by FOXO3, which is why FOXO3 staves off aging.
While FOXO3 is activated by caloric restriction and intermittent fasting (NOTE: IGF-1 is the major inhibitor of FOXO3 and the nutritional regulation of IGF-1 will be discussed in a future article). Other dietary activators of FOXO include EGCG found in green tea and quercetin found in yellow onions.
Heat stress, such as from using the sauna, also activates FOXO3 as well as other mediators of stress tolerance known as heat shock proteins, which I have discussed in detail in another video. The sauna is a great example of using stress (in the form of heat stress) to activate genetic pathways that help the body deal with a variety of stresses.
Telomeres
Telomeres are also associated with longevity and are epigenetically regulated by environmental factors. Every cell in your body contains DNA, which is present in your chromosomes, and the integrity of your DNA is crucial for your cells to function properly. Telomeres are caps at the end of chromosomes that protect your DNA from damage and deterioration.
Every time a cell divides in your body, it must copy all the DNA inside the cell, including the telomere DNA. The problem is there is a structural defect in the telomere DNA so that a little piece of the telomere DNA does not get copied because enzymes that copy the telomere DNA cannot access it (Figure 3). So why is this important? In your body, every time a cell divides, your telomeres get shorter with each cell division. On average we lose about 21 nucleotides (structural units ) off our telomeres per year until there are no telomeres left, your cells either become senescent or they die. This is why the length of your telomeres is like a biological clock, it is a marker for aging.
There are several factors that accelerate the shortening of telomeres including DNA damage, inflammation, lack of sleep, obesity, poor nutrition, and more. It comes as no surprise that both vitamin D and omega 3, which lower DNA damage and inflammation, have been shown to slow telomere shortening. In one study involving 2,100 female twins, those with the lowest vitamin D levels had shorter telomeres that corresponded to 5 years of accelerated aging.
Exercise slows telomere shortening by lowering overall inflammation. In a study involving 2,400 twins, those who were less physically active had shorter telomeres than those who were more active, and the most active subjects had telomeres the same length as sedentary individuals up to 10 years younger, on average.
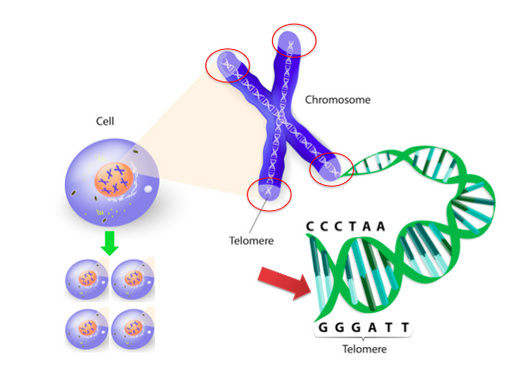
Figure 3: Telomeres Protect DNA
Telomeres are tiny caps and the end of chromosomes that protect DNA from damage. When a cell divides, it must replicate all the DNA, including telomere DNA
Summary
In summary, we all have gene polymorphisms that may have downsides to them that may be ameliorated by different micronutrients interventions. The first thing to do is to become aware there are tools out there that allow you to understand your own gene polymorphisms better. For example, you can get a 23andMe genetic test and run it through promethease and it will give you some information about what your gene polymorphisms mean. Keep in mind these tools are not perfect and there are many interactions between gene polymorphisms so just because you may have a particular polymorphism that may have a loss of function, you may have other polymorphisms that compensate. In addition, we discussed epigenetics including how diet and lifestyle factors can changes the expression of genes in a good and bad way and how this can be passed on to children, how epigenetic marks change with age, and what lifestyle factors can reverse or prevent this.
Related:
