I am talking about increasing glucagon not decreasing it! If I am fasting extensively for more than ten days and I eat a little piece of protein let’s say a quarter ounce chunk of meat, that is going to throw me into a fed state and out of autophagy? (not likely, anomalous and brief at best)
Eating that little piece of protein is the mTORC1 ON/OFF (balance) switch or window. Glucose, ketones and free fatty acids all inhibit glucagon release. Amino acids stimulate glucagon release and uptake into the liver, covalent phosphorylation (NADH + (1/2)O2+ H+ ==>NAD+ + H2O = redox potential) initiated by glucagon activates the former and inhibits the latter.
EF/IF FASTING==>DELTA SLEEP==>HGH==>IGF-1, IGF-2, EGF (or other variable)==>OFF[mTORC1]ON<==GLUCAGON<==PROTEIN INTAKE
Fung: “…Is it all protein or certain amino acids? The answer is not known. In animal studies the specific amino acid that is critical differs between species. In humans, branched chain amino acids SEEM to be a particularly strong activatory of mTOR… …More
Then this is stated:
Fung: “…What turns off autophagy? Eating. Glucose, insulin (or decreased glucagon) and proteins all turn off this self-cleaning process. And it doesn’t take much. Even a small amount of amino acid (leucine) COULD stop autophagy cold. So this process of autophagy is unique to fasting – something not found in simple caloric restriction or dieting. There is a balance here, of course. You get sick from too much autophagy as well as too little. Which gets us back to the natural cycle of life – feast and fast. Not constant dieting. This allows for cell growth during eating, and cellular cleansing during fasting – balance. Life is all about balance. …More
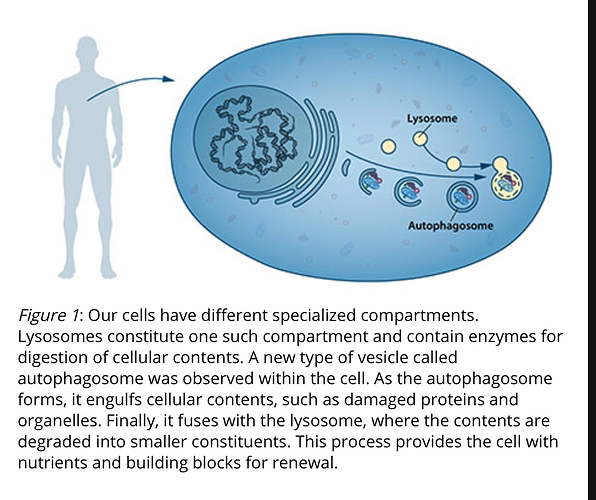
(Figure 1). Christian de Duve, the scientist behind the discovery of the lysosome, coined the term autophagy, “self-eating”, to describe this process. The new vesicles were named autophagosomes. …More
Insulin and Glucagon
Effects of glucagon and fasting on acetate metabolism in perfused rat liver Abstract (1) Effects of glucagon treatment in vitro and of fasting on [2-14C] acetate metabolism were studied in perfused rat liver. Glucagon stimulated ketogenesis about 2.8 fold and the incorporation of label into ketone bodies only 1.7 fold. The diminished specific activity of ketone bodies produced in the presence of glucagon was interpreted as evidence for increased production and disposal (turnover) of acetyl-CoA. Inhibition of acetyl-CoA utilization by other routes, therefore, was not a major cause of the increased ketogenesis. From a consideration of label incorporation into derivatives of the Krebs cycle in relation to the specific activity of acetyl-CoA (as judged from ketogenic rate and label incorporated into ketone bodies) glucagon appeared to accelerate the initial steps of the Krebs cycle about 2 fold. It diminished label incorporation into fatty acids to a degree corresponding to the reduction in acetyl-CoA specific activity. Incorporation of label into cholesterol was not affected by glucagon. (2) In livers from fasting rats, ketogenesis was increased about 6.7 fold and label incorporation into ketone bodies was increased about 5.3 fold over values in fed controls. Thus, the turnover of acetyl-CoA appeard to be increased slightly by fasting. Krebs cycle activity was essentially unaffected by fasting. The inhibition of fatty acid and cholesterol synthesis in these livers appeared sufficient to contribute significantly to the ketogenic rate by sparing acetyl-CoA. (3) Gluconeogenesis from endogenous substrate was markedly stimulated by glucagon and moderately stimulated by fasting.
(4) Secretion of newly synthesized total fatty acids, cholesterol and cholesterol ester into the circulation was markedly inhibited by glucagon.
Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin There is evidence that the activity of mTOR in cells is increased by amino acids (22–24), and the protein is believed to function in a nutrient-sensing pathway that maintains a proper balance between amino acid availability, protein synthesis, and cell growth (16, 17).
Glucagon Decreases IGF-1 Bioactivity in Humans, Independently of Insulin, by Modulating Its Binding Proteins.
mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate
Scientists find off-switch for the mTor complex
The important control functions of the mTor kinase influence cell metabolism, division and growth. For example, the molecular control unit ensures the production of new proteins or the storage of fat and carbohydrates in metabolically active tissue. These processes are stimulated by the influx of sugar and amino acids and by signals initiated by growth factors including the insulin-like growth factor. If this influx does not occur in a starvation period, mTor switches the cell from anabolic to catabolic mode. Instead of synthesizing new proteins from amino acids, the cell now activates clean-up processes to remove damaged proteins, which could become hazardous for the cell or organ. The cleansing effect of interval fasting is attributed to the consequences of deactivating the mTor kinase.
As the cell’s molecular control center, the mTor kinase regulates cellular metabolism, growth and division. However, in cells affected by pathological change, the regulation goes array. Therefore, it would be helpful if the central control could be simply turned off to suppress insulin resistance or cancerous growth for example. Scientists at the Leibniz–Forschungsinstitut für Molekulare Pharmakologie (FMP) in Berlin (Germany) succeeded in locating a crucial off-switch for the central cell control. Paradoxically, this ‘off-switch’ is a lipid kinase producing a product previously known for its role in the activation of mTor. The results just appeared in the high-ranking journal Science. They bolster the hopes of patients waiting for new effective therapies against diabetes, obesity, cancer and a rare congenital muscular disease.
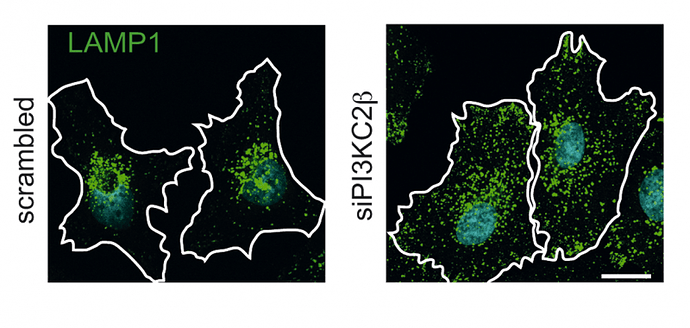
As the scientist discovered, another scarcely investigated specific class II lipid kinase (PI3KC2ß) exists in the cell, which deactivates the mTor complex on the lysosomal membrane in the absence of stimulating signals from the outside. In the absence of hormonal signals such as insulin or insulin-like growth factor (e.g. at night), the lipid kinase PI3KC2ß becomes active at the lysosomal membrane. The active lysosomal lipid kinase phosphorylates a lipid, which then deactivates the mTor complex. Under these conditions the lysosome mainly functions to degrade cellular proteins.…More
Autophagy: cellular and molecular mechanisms
Analog modeling of glucagon-induced autophagy in rat liver. I. Conceptual and mathematical model of telolysosome-autophagosome-autolysosome interaction.
Scientific Illuminism by Paul Joseph Rovelli Liber Vox Viva Voce vel Video pp. 118: “…Overall, energy moves from the physical and etheric bodies to the emotional and then subsequently to the mental to the spiritual bodies and simultaneously, in the opposite direction as well, culminating in the physical body.
”…For each cell, if you separate the two strands of DNA, you will discover a connecting link, a ‘phosphorylation’ or ‘fizz of light’ (Hadit) that is the connecting current of the two strands.…” “…Remember that DNA is but a protein and it is this light or phosphorylation that regulates its activity by attaching it to a phosphate group, such as ATP, which causes a structural change in the protein to enable it to bind or release itself to or from some other molecule. This is important when considering the cell’s need for oxygen. The cell is either reduced or negatively charged (reduction), being in need of oxygen or it is oxidized and positively charged (oxidation). A unit with two cells, one in each of these two states is called an electrochemical half-cell. Together, they will seek a state of electro-chemical equilibrium relative to at least one common intermediate half-cell, giving us a voltage.
This system is referred to by scientists as ‘redox’ and is further described by the following formula: NADH + (1/2)O2+ H+ ==>NAD+ + H2O
In their paper on Quantum Bioholography, Richard & Iona Miller note:
“The superposed coherent waves of different types in the cells are interacting to form diffraction patterns, first in the ‘acoustic’ domain and in the electromagnetic domain. This is a kind of quantum hologram. Interactions of solitonic oscillations in the liquid crystal structure of DNA and the polarization vector of the ultra weak biophotonic highly coherent light, could be understood as a mechanism of translation between holograms in the ‘acoustic’ frequency domain of short range effects and those in the electromagnetic domain, and vice versa.”
The “light” they are referring to seems to parallel Eliphas Levi’s AUR with the “acoustic” energy equivocating with the AUD and the electromagnetic energy equivocating with the AUB.
NADH is ‘Nicotinamide adenine dinucleotide,’ a coenzyme made from vitamin B2, or niacin. It’s a coenzyme present in all living cells and serves an important role in helping enzymes to function as they should. An enzyme is a protein that works as a catalyst to generate chemical changes in other substances, such as breaking down food into energy. NADH stimulates the production of ATP (adenosine triphosphate), which is a compound that regulates the release of energy stored in cells. The more NADH a cell has, the more chemical energy it produces. Increased concentrations of NADH in the brain may even boost the production of neurotransmitters so vital to sound mental function.