NB: This topic has been configured as a wiki - so anyone can update the original post with ideas. Just click on the green edit icon ![]() on the top right of the post.
on the top right of the post.
Fats and Oils are a complex subject. But I’ll have a crack at explaining it. Just a little biochemistry tho … sorry.
So the chemical structure of a fat looks a bit like this, a chain of carbons ©, with legs made of hydrogens (H) and a funky bit down the right hand end with the double bonds and the oxygens (O) and that is the bit that makes this molecule an acid.
Butyric Acid
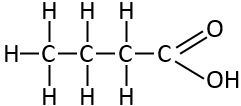
This one is one of the shortest in our diet and it’s the one that gives butter and cinema popcorn its unique smell.
This is known as a saturated fatty acid because every carbon is linked to its neighbours by a single bond which makes it rigid and solid at room temperature. It also doesn’t go rancid easily.
Butyric acid is It’s known as C4:0 - because it has 4 carbons and none of the carbons are joined with a double bond (just the carbon oxygen bond at the right hand end).
All fats look kind of the same the biggest difference is how long that spine of carbons is, and whether any of those carbon to carbon bonds are single or double bonds.
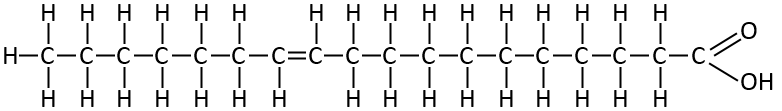
Caprylic Acid
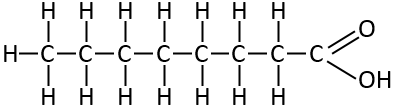
This is one of the Medium Chain Fatty acids. It’s the primary one in Dave Asprey’s Brain octane MCT product. And you find it in Coconut oil, and goats milk.
Notice it’s just the same as the previous one but longer. This one is known as C8:0. It’s just the same pattern. A saturated fat with every carbon linked by single bonds.
Now you probably wonder about unsaturated fatty acids. Well they look similar except one or more of the links between the carbons has a double bond and the entire chain has one or more kinks to it. You can see how that kink affects the properties of the fat because that kink allows it to be more flexible and liquid at room temp.
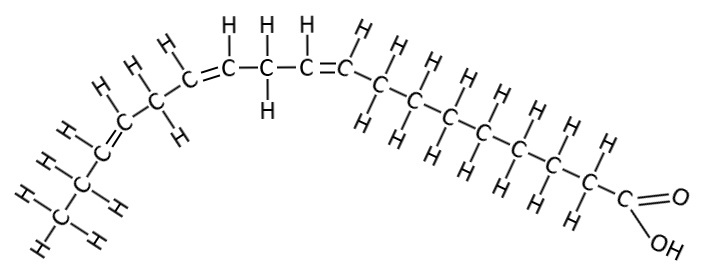
Oleic Acid
This one is the monounsaturated fatty acid common in Olive oil, and also commonly stored in human body fat cells.
This one is known as C18:1 omega cis9 - which is shorthand for a fat with 18 carbons, 1 double bond at position 9 and both the hydrogens are on the same side. It’s the same pattern again but this time it’s a little kinky around the 9th carbon bond from the left, where it has a double bond and both carbons only have one hydrogen (H) leg on the same side of the spine and so it’s bendy.
While I’m at it I’ll just mention a polyunsaturated fatty acid which will have more than one double bond
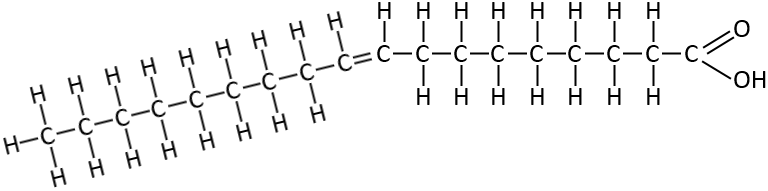
alpha-Linolenic acid
This one is an omega 3 polyunsaturated fatty acid found in chia, flax and nuts. It is also one of the essential fatty acids that we have to eat and can’t manufacture internally.
Notice that is has multiple double bonds, which makes it a polyunsaturated fatty acid. The first is at position 3 then at 6 and then at 9. The position of the first double bond is it’s omega value. This is an omega 3 polyunsaturated fatty acid.
Sidebar: You might wonder why plants seem to mostly use unsaturated oils and animals use mostly saturated fats.
Well it turns out that saturated fats are much more stable. They have fewer carbon-carbon double bonds that can become oxidized (ie: go rancid). Saturated fats are also not normally liquid at room temperature.
Animals however run at a hotter temp than the background environment so they are able to move saturated fats around in liquid form.
Plants of course are at the environment background temperature, so they have to use the more volatile unsaturated fats.
It’s not always the case tho. Animals do use some unsaturated oils - for example our primary storage forms of fat are the saturated fat Palmitic acid and the monounsaturated fat Oleic acid. And some plants, especially tropical ones like Coconuts are able to use the shorter chained saturated fats.
So there are two kinds of carbon to carbon double bonds in organic chemistry.
##Cis bond
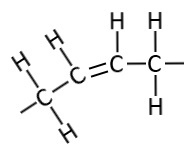
We’ve seen the CIS bond before in the mono and polyunsaturated fatty acids. The 2 carbons either side of the double bond have only one Hydrogen each and it’s on the same side of the spine. Those 2 hydrogens have a positive charge so they repel each other and the molecule bends at this position in it’s chain.
##Trans bond
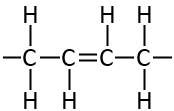
In the trans bond however, those 2 hydrogens are on opposite sides to the molecule ends up being quite straight. This rigidity means that these molecules are able to be quite solid even with a double bond.
You’ve probably heard about Trans fats and they are made by hydrogenating seed oils. Well that alpha-Linolenic acid is an example of a seed oil, and hydrogenation is the process of adding hydrogens to it to make it behave like a saturated fat and be solid at room temp. Think margarine.
Vaccenic acid
There are also naturally occurring trans fats such as this one which is in small amounts in human breast milk, and in beef meat.
The reason that trans fats are not good to eat in large amounts is because of what we do with unsaturated fats in our body. We use them to build the lipid membrane around our cells. If we have nice bendy polyunsaturated fats like alpha-Linolenic acid then we retain flexible cell membranes. If however we have trans fatty acids then we end up with rigid inflexible cell membranes.