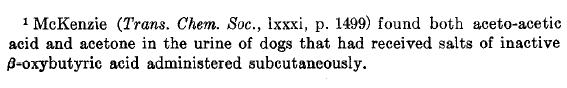Endogenous production D-BHB & L-BHB:
This is a super complex subject so this might be merely speculatory?
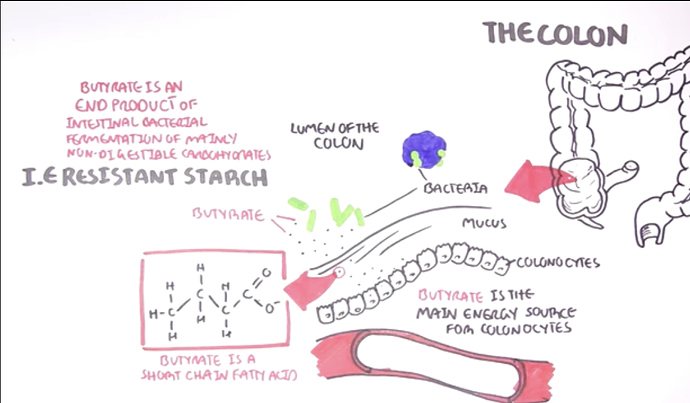
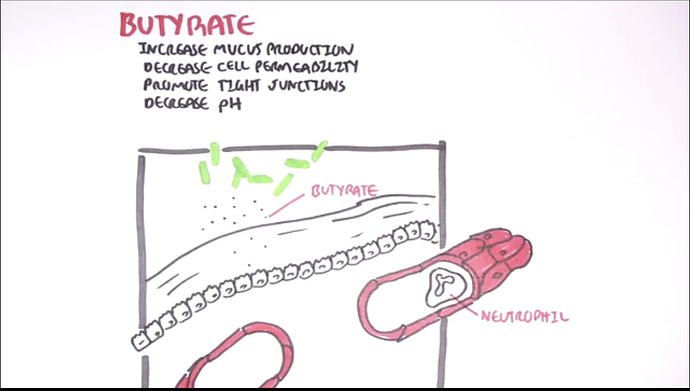
The very ending word “butyrate” means BUTTER or the short chained fatty acids that certain gut bacteria make and ferment when you eat Resistant Starch e.g. HAM:RS2 or when you eat GRASS FED butter.
image source
This endogenous production of D-BHB & L-BHB may revolve around butyric acid, fermentation in the gut in contrast to INSULIN secretions and dipping into unexplored territory?
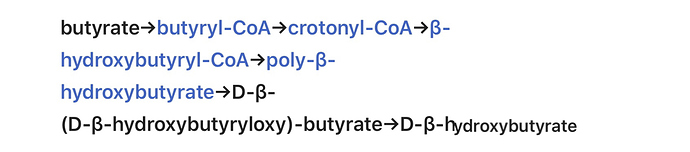
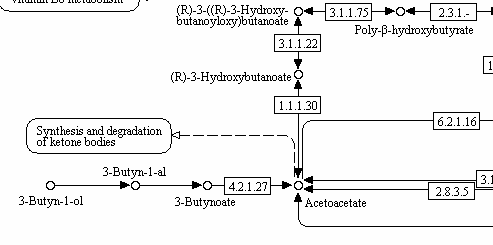
Pathway of D-BHB:
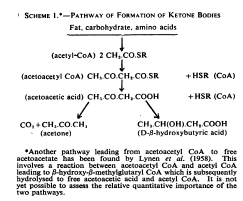
butyrate→butyryl-CoA→crotonyl-CoA→β-hydroxybutyryl-CoA→poly-β-hydroxybutyrate→D-β-(D-β-hydroxybutyryloxy)-butyrate→D-β-hydroxybutyrate[4]
Pathway of L-BHB: ?
Related:
[1] Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes.
[2] “…High anion gap. The anion gap is affected by changes in unmeasured ions. In uncontrolled diabetes, there is an increase in ketoacids due to metabolism of ketones. Raised levels of acid bind to bicarbonate to form carbon dioxide through the Henderson-Hasselbalch equation resulting in metabolic acidosis. …” …More
[3] Relationship Between Hydroxybutyrate and Acetoacetate Plus Acetone Contents of Blood and Urine of the Ruminant: Animals: Experimental Procedure: Mature female Saanen goats were used as experimental animals throughout the studies. To create states of hyperketonemia, goats were infused with butyric acid during metabolic states characterized by hypoglycemia. The hypo- glycemia was created by phlorizin treatment or by fasting the goats in late pregnancy. Details of the experimental methods have been published (6, 7). …” “…In the present study, more of the values for both the blood and urine ketone fractions were within the normal physiologic range than in Reid’s study (8), which might account for the differences noted. Based on our data, it would appear that above a blood or plasma concentration of B-hydroxybutyrate or acetoacetate plus acetone of 2 mg acetone/100 ml, a large increase in urinary concentration or excretion of the respective fractions occurs. Most work with monogastric animals indicates that both acetoacetate and B-hydroxybutyrate have urinary threshold levels (5, 14, 15), while acetone does not (15). The present study indicates that both urinary B-hydroxybutyrate and acetoacetate and acetone excretion increase with increasing blood or plasma levels, and above a whole blood or plasma concentration of 2 mg /100 ml, expressed as acetone, large increases in urinary concentration and excretion of both ketone fractions occur. At low concentrations of ketones in either blood or urine, B-hydroxybutyrate appears to be the major ketone body. Similar data obtained by Schultz and Myers (10) and Thin and Robeison (12) with cows showed a de crease in whole blood or plasma B-hydroxybutyrate :acetoacetate plus acetone ratios with increasing total ketone body concentration. In the present study, the de- crease in this ratio in whole blood, plasma, or urine occurred below total ketone body concentrations of 4 mg acetone/100 ml, and the ratio plateaued at a value of 2-3 above this concentration. The reason for this decreased ratio remains to be clarified, although it may be related to a decreased carbohydrate utilization due to hypoglycemia, which characterized the ketotic states reported in this study (6, 7). …More
[4] “…Butyrate can also be metabolized into D-β-hydroxybutyrate via a second metabolic pathway that does not involve acetoacetate as a metabolic intermediate. This metabolic pathway is as follows:[3]
The last reaction in this metabolic pathway, which involves the conversion of D-β-(D-β-hydroxybutyryloxy)-butyrate into D-β-hydroxybutyrate, is catalyzed by the hydroxybutyrate-dimer hydrolase enzyme.[3] …More