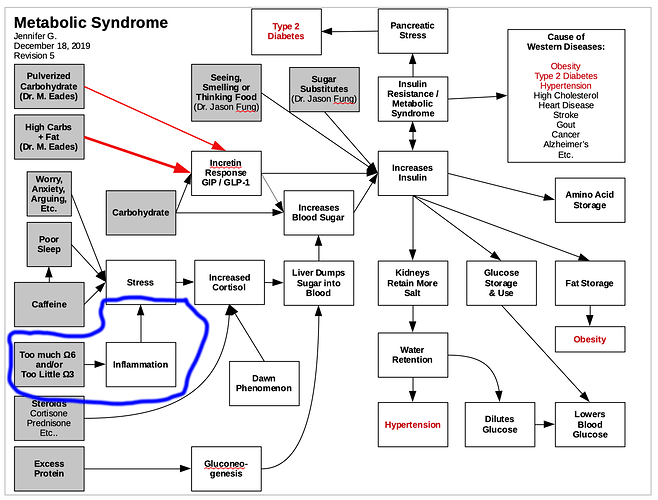
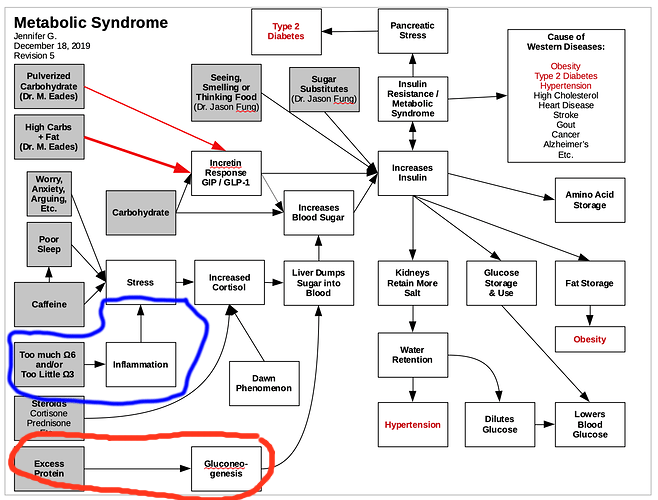
Does 50–60% of protein become glucose and enter the bloodstream in 3–4 hours?
…
This raises the question of why, if gluconeogenesis from protein occurs, does the glucose produced not appear in the general circulation? Several theories have been suggested. The first is that considerably less than the theoretical amount of glucose (50–60%) produced from protein actually is produced and enters the general circulation, and the small amount of glucose released is matched by a corresponding increase in glucose use, if adequate insulin is available.4 Another theory suggests that the process of gluconeogenesis from protein occurs during a 24-hour period, and the slowly and evenly produced glucose can be disposed of over a long period of time.5 It is also speculated that the insulin stimulated by dietary protein causes the glucose formed to be rapidly stored as glycogen in the liver and in skeletal muscles. This glucose can then be released when insulin levels are low or glucagon levels are elevated, and the body does not identify if the glucose is from protein or carbohydrate.
To understand this process of gluconeogenesis and the question of why protein does not affect blood glucose levels, it is helpful to briefly review the metabolism of dietary proteins. The majority of protein is digested, and the amino acids not used for gut fuel are metabolized in the intestinal mucosal cells and transported by the portal vein to the liver for protein synthesis or gluconeogenesis.12 In the liver, nonessential amino acids are largely deaminated, and the amino group (nitrogen) removed is converted into urea for excretion in the urine.13 It has been shown that in subjects without and with mild type 2 diabetes, ~50–70% of a 50-g protein meal is accounted for over an 8-hour period by deamination in the liver and intestine and synthesis to urea.14 It has been assumed that the remaining carbon skeletons from the nonessential amino acids are available for glucose synthesis, which would then enter into the general circulation.
The essential amino acids pass through the liver into the general circulation, where they may be removed and used for new protein synthesis or, alternatively, for skeletal muscle fuel. Circulating amino acids stimulate insulin and glucagon secretion. The amino acids that stimulate glucagon are different from those that stimulate insulin secretion.15-17
To add to the confusion, the effect of protein on glucose appearance is influenced by insulin availability. With insulin deficiency, the oxidation of branched chain amino acids in muscle and uptake of alanine (the principle glycogenic amino acid) by the liver is accelerated, resulting in increased gluconeogenesis and augmented protein catabolism.18 The accompanying rise in glucose levels is most likely due to an increased conversion of ingested protein into glucose and to a decreased glucose removal rate. In subjects with diabetes who had insulin withheld for 24 hours, there was a three- to fourfold increase in liver glucose output after protein ingestion.19 However, in the presence of insulin, alanine uptake by the liver is virtually zero,20 and hepatic glucose production falls by 85%.21 Indirectly then, insulin could reduce gluconeogenesis in the liver by decreasing the amino acid substrate supply. Insulin also inhibits the degradation of body proteins and lowers the circulating concentration of many amino acids.22
The net effect on glucose output by the liver depends on the ratio of insulin to glucagon. In people with type 1 or type 2 diabetes, the glucagon response to protein is considerably greater than in people without diabetes.4 Glucagon stimulates an increase in hepatic glucose production due to an increase in glycogenolysis and an increase in gluconeogenesis. Glucagon antagonizes the effect of insulin in the liver. However, it does not antagonize the insulin-stimulated uptake of glucose in muscle or the insulin-mediated decrease in release of non-esterified fatty acids from fat cells.4
Therefore, the process of gluconeogenesis is affected by substrate supply and level of glycemic control. However, in people with well-controlled diabetes, minimal amounts of hepatic glucose are released into the general circulation after the ingestion of protein.
...
References
4 Gannon MC, Nuttall FQ: Protein and diabetes. In: American Diabetes Association Guide to Medical Nutrition Therapy for Diabetes. Franz MJ, Bantle JP, Eds. American Diabetes Association, Alexandria, Va., 1999, p. 107-25.
5 Conn JW, Newburgh LH: The glycemic response to isoglucogenic quantities of protein and carbohydrate. J Clin Inves t 15:667-71, 1936.
6 Nuttall FQ, Gannon MC: Plasma glucose and insulin response to macronutrients in nondiabetics and NIDDM subjects. Diabetes Care 14:824-38, 1991.
7 Westphal SA, Gannon MC, Nuttall FQ: The metabolic response to glucose ingested with various amounts of protein. Am J Clin Nutr 52:267-72, 1990.
8 Khan MA, Gannon MC, Nuttall FQ: Glucose appearance rate following protein ingestion in normal subjects. J Am Coll Nutr 11:701-706, 1992.
9 Krezowski PA, Nuttall FQ, Gannon MC, Bartosh NH: The effect of protein ingestion on the metabolic response to oral glucose in normal individuals. Am J Clin Nutr 44:847-56, 1986.
10 Nuttall FQ, Mooradian AD, Gannon MC, Billington CJ, Krezowski PA: Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 7:465-70, 1984.
11 Gannon MC, Damberg G, Gupta V, Nuttall FQ: Ingested protein has little effect on glucose concentration or rate of glucose appearance in people with type 2 diabetes [Abstract]. J Am Coll Nutr 18:546 (Abstract no. 97), 1999.
12 Nuttall FQ, Gannon MC: Plasma glucose and insulin response to macronutrients in nondiabetics and NIDDM subjects. Diabetes Care 14:824-38, 1991.
13 Windmueller HG, Spaeth AE: Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem 249:5070-79, 1978.
14 Nuttall FQ, Gannon MC: Metabolic response to dietary protein in people with and without diabetes. Diab Nutr Metab 4:71-88, 1991.
15 Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J: Insulin secretion in response to protein ingestion. J Clin Invest 45:1479-86, 1966.
16 Rabinowitz D, Merimee TJ, Maffezzoli R, Burgess JA: Patterns of hormonal release after glucose, protein, and glucose plus protein. Lancet ii :454-57, 1966.
17 Muller WA, Faloona FR, Aquilar-Parada F, Unger RH: Abnormal alpha-cell function in diabetes: response to carbohydrate and protein ingestion. N Engl J Med 28:109-15, 1970.</p<
18 Felig P, Wahren J, Sherwin R, Palaiologos G: Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med 137:507-13, 1977.
19 Wahren J, Felig P, Hagenfeldt L: Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes. J Clin Invest 57:987-99, 1976.
20 Felig P, Wahren J, Hendler R: Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes 24:468-75, 1975.
21 Felig P, Wahren J: Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest 50:1702-11, 1971.
22 Zinnerman HH, Nuttall FQ, Goetz FG: Effect of endogenous insulin on human amino acid metabolism. Diabetes 15:5-8, 1966.
23 Wolever TMS, Nguyen P-M, Chiasson J-L: Determinants of the diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 59:1265-69, 1994.
24 Nordt TK, Besenthal I, Eggstein M, Jakober B: Influence of breakfasts with different nutrient contents on glucose, C peptide, insulin, glucagon, triglycerides, and GIP in non-insulin-dependent diabetics. Am J Clin Nutr 53:155-60, 1991.