Some of my notes:
I think Gamma delta T cells are unique in blocking the path in for CD147 with covid as it will allow Gamma delta T cells to do it job and a low carb diet may be the only way to increase them higher than baseline.
The phenotypic changes of γδ T cells in COVID-19 patients
Abstract
A novel pneumonia-associated respiratory syndrome named coronavirus disease-2019 (COVID-19), which caused by SARS-CoV-2 and broken in Wuhan, China in the end of 2019. Unfortunately, there is no specific antiviral agent or vaccine available to treat SARS-CoV-2 infections. Also, information regarding the immunological characteristics in COVID-19 patients remains limited. Here we collected the blood samples from 18 healthy donors (HD) and 38 COVID-19 patients to analyze changes in γδ T cells. In comparison to HD, the γδ T cells percentage was decreased. γδ T cells are able to immediately respond to SARS-CoV-2 infection and upregulate the activation marker CD25. In addition, the increased expression of CD4 in γδ T cells may serve as a biomarker for the assessment of SARS-CoV-2 infection.
From <https://www.medrxiv.org/content/10.1101/2020.04.05.20046433v1
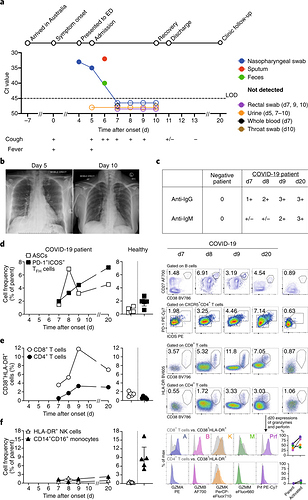
SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion
https://www.nature.com/articles/s41423-020-0424-9.pdf
This was the Gamma T Cell study Keto mice
which said "These data thus indicate that KD promotes the enrichment of a subset of lung gd T cells with a unique genetic signature distinct from those induced by HFD."
But it wasnt ketone related but more fax oxidation related "Given that BHB serves as the primary energy substrate during glucose deprivation and KD feeding (27), we next tested whether BHB itself was responsible for the gd T cell increase in the lungs after IAV infection. To test this possibility, we fed mice 1,3-butanediol (BD), which increases circulating BHB levels without the requirement for mitochondrial fatty acid oxidation, a necessary metabolic switch induced by KD. Although BD efficiently elevated blood BHB to levels comparable with KD-fed mice, it failed to induce gd T cell expansion"
Mice fed a ketogenic diet—in which 90 percent of calories come from fat and less than 1 percent from carbohydrates—were less susceptible to the influenza A virus, according to a study published today (November 15) in Science Immunology. The protective effects seem to be mediated by an increase of so-called gamma-delta T cells in the animals’ lungs that induce the epithelial cells in the airway to make more mucus to trap the virus.
They started by keeping mice on a normal diet, in which 18 percent of calories come from fat and 58 percent from carbs, or feeding them a ketogenic diet for seven days, and then infecting them intranasally with H1N1 influenza. All animals eating the normal diet were dead by the fourth day after infection, while half of the mice fed the keto diet survived.
Humans and mice have two types of T cells: alpha-beta T cells, which are well studied and specific to pathogens, and gamma-delta T cells, which detect signs of stress, are more numerous in the lungs, skin, and gut, and are much less well understood. The researchers found that mice fed a ketogenic diet have about four times the number of gamma-delta T cells in the lungs as mice fed a normal diet by the third day after infection. Then they analyzed gene expression in flu-infected lungs from mice fed the keto diet and determined that the gamma-delta T cells were protecting the mice in a surprising way.
“Typically, T cells are thought to control viral infection by killing the infected cells. What we found was that instead of killing, gamma-delta T cells modify the environment . . . to protect the host against the virus,” writes Iwasaki in an email to The Scientist. “These T cells modify the airway epithelial cells to produce more mucus that can trap the virus in its tracks.”
These two ideas—that a diet that the mice are being fed contributes to protection by improving barrier function and that the mechanism of that protection is being mediated by gamma-delta T cells—haven’t received much attention in influenza biology, says Paul Thomas, an immunologist at St. Jude Children’s Research Hospital who did not participate in the study. “Why are gamma-delta T cells an effector of a diet-induced changed in metabolism” is one open question, he adds, and another is “whether or not the same pathways exist in humans, and if they do, how that might affect how we handle influenza infection.”
The findings indicate that the keto diet could have similar protective effects in people.
Obese and diabetic patients incur more hospitalizations and have increased severity of influenza infections each year,” Julie Jameson, an immunologist at California State University, San Marcos, who did not participate in the work, writes in an email to The Scientist. It’s already known that a ketogenic diet can help these patients lose weight and improve their cholesterol levels and blood pressure, she adds, and this study raises the possibility that it “may have even more health benefits than previously reported.”
According to Jameson, it is still not clear what the consequences of a long-term ketogenic diet are. “This study examines a one-week, acute time point. It is important to know whether gamma-delta T cells would continue to accumulate in the lung and whether this would be beneficial,” she explains.
From <https://www.the-scientist.com/news-opinion/keto-diet-protects-mice-from-flu-66731>
Gamma delta T cells Powerful
A collaborative group of scientists led by investigators at the University of Birmingham have just identified a novel role for an enigmatic and unconventional subset of immune cells known as gamma delta T cells (Vδ2 T cells). The researchers examined how this subtype of T cell responded to a virus infection called cytomegalovirus. They found that when these T cells detected signs of the virus infection, they both increased in numbers and became “licensed to kill.” Findings from the new study were published today in Nature Communications (“The Human Vδ2+ T-Cell Compartment Comprises Distinct Innate-Like Vγ9+ and Adaptive Vγ9– Subsets”).
We think these cells contribute to defense against viral infection in the liver, a site that is exposed to many potentially dangerous infectious diseases,” Dr. Davey noted. “They may also be particularly important when other aspects of our immune system are not working at full strength, such as in newborn babies, but also in transplant patients who are taking immunosuppressive drugs to prevent organ rejection.
Gamma delta (γδ) T cells are the prototype of ‘unconventional’ T cells and represent a relatively small subset of T cells in peripheral blood. They are defined by expression of heterodimeric T-cell receptors (TCRs) composed of γ and δ chains. This sets them apart from the classical and much better known CD4+ helper T cells and CD8+ cytotoxic T cells that express αβ TCRs. The mechanism of (thymic) selection of γδ T cells is still largely unkown.
From <https://www.immunology.org/public-information/bitesized-immunology/cells/gamma-delta-γδ-t-cells>
The same group (53) further analyzed the antiviral functions of the Vδ2 T-cells on Huh7 hepatoma cells carrying the subgenomic HCV replicon. Activation of the Vδ2 cells was associated with a marked reduction of HCV RNA levels. The neutralization of IFN-γ by antibodies revealed the importance of this cytokine in inhibiting HCV replication. The inhibition of HCV was increased by the use of aminobisphosphonates that activate γδ T-cells – thus, pointing toward a potential future immunotherapeutic strategy. These studies suggested that depending on the type of Vδ chain expressed by the γδ T-cells, the cells might either have a beneficial or deleterious effect in patients with chronic HCV infection.
From <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4143964/>
Increased CD147 Expression in Cancer Cells Is Associated with Decreased Overall Survival
From <https://www.hindawi.com/journals/bmri/2015/242437/>
CD147 expression was found in 112 out of 129 tumor entities with the following incidences: squamous cell carcinomas (60-100%), pancreatic (87%), chromophobic kidney (83%), hepatocellular (83%), medullary breast (83%) and glioblastoma multiforme (79%).
From <http://cgp.iiarjournals.org/content/7/3/157.full>
Treatment of transplant patients with a CD147 antibody was effective due to inhibition of T-cell activation
From <http://cgp.iiarjournals.org/content/7/3/157.full>
Gamma delta T cells killing cancer cells
From <https://www.youtube.com/watch?v=v2tphZPOabY>
Don’t belong to CD4 and CD8
GAMMA-DELTA T CELLS
T cells expressing the gamma-delta (γδ) TCR represent the third population of nonclassical T cells in humans. The main characteristic of human γδ T cells is their expression of unique TCR heterodimers. Three main populations of γδ T cells are detected: The most abundant population in circulating blood and secondary lymphoid organs expresses the Vδ2 chain almost always paired with the Vγ9 chain. These TCR heterodimers are disulphide linked as they use the Cγ1 constant region and are polyclonal. Junctional diversity is present in the CDR3 regions of both the TCR γ and δ chains. The second main population expresses the Vδ1 chain, which is paired with different Vγ chains, mostly belonging to the VγI family (Vγ2, 3, 4, 5, and 8). This population of γδ T cells is polyclonal and the TCR is not disulphide linked, as in most of the cases the Cγ2 constant region is used. The Vδ1-expressing cells are abundant in the lamina propria of the gut and in the intraepithelial spaces. Noteworthy, these cells are also found in the lung and in the skin. The third population of γδ T cells expresses the Vδ3 chain, which usually pairs with Vγ chains of the VγI family.
Antigen Recognition by γδ Cells
Although we know a lot about TCR gene use, tissue localization, and effector functions of γδ T cells, we remain quite unaware of the antigens that stimulate human γδ T cells. In addition, we have only partial information on the function of this T-cell population in TB.
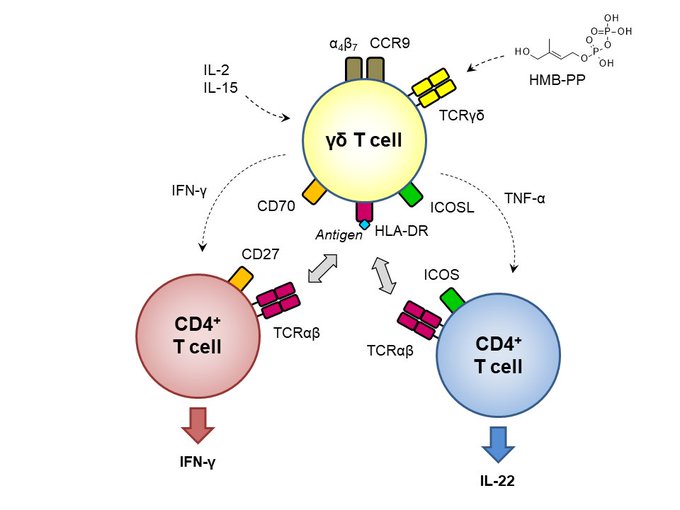
The population expressing the TCR Vγ9-Vδ2 heterodimer recognizes phosphorylated metabolites, such as isopentenyl pyrophosphate (IPP) and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), which are produced in the mevalonate and methyl erythritol pathways, respectively (Fig. 2B). IPP is made by eukaryotic and prokaryotic cells, whereas HMBPP is made only by some microorganisms, including MTB. IPP and HMBPP are very small molecules (245 and 262 Da, respectively) and require appropriate antigen presentation. Recently, it was reported that Butyrophilin 3A1 (BTN3A1) is required for activation of Vγ9-Vδ2 cells (Harly et al. 2012) and that this molecule is involved in the presentation of both phosphorylated metabolites to the TCR Vγ9-Vδ2 (Vavassori et al. 2013). Surface plasmon resonance and surface-enhanced Raman scattering showed a direct interaction of the TCR Vγ9-Vδ2 with BTN3A1, and the presence of IPP increased the binding (Vavassori et al. 2013). Studies performed with site-directed mutagenesis of TCR chains indicated that the TCR becomes activated only when several amino acids present in the CDR regions of both the TCR γ and δ chains interact with the antigen-presenting molecule (Wang et al. 2010).
Interestingly, BCG-infected cells stimulated only a subset of Vγ9-Vδ2 cells, and only a fraction of Vγ9-Vδ2 cells expanded in vitro with synthetic HMBPP was reactive to infected cells (Spencer et al. 2008). Whether the differential response results from recognition of additional mycobacterial antigens or reflects differences in TCR avidity or variable expression of co-stimulatory/inhibitory molecules remains to be investigated.
The antigens stimulating γδ cells expressing non-Vγ9 chains and non-Vδ2 chains remain poorly characterized. These cells can be activated during CMV infections, and one clone expressing a Vδ5-Vγ4 TCR was activated by the endothelial protein C receptor (Willcox et al. 2012). Vδ1-expressing cells were also found to interact with the bacterial phycoerythrin protein in a manner resembling antigen–antibody recognition, without requirements for antigen processing or presentation by dedicated presenting molecules (Zeng et al. 2012).
A small number of non-Vδ2 cells recognize glycolipids presented by CD1c and CD1d molecules (Faure et al. 1990; Spada et al. 2000; Bai et al. 2012). The structure of the TCR γδ-CD1-lipid complex showed a MHC-like recognition (Luoma et al. 2013; Uldrich et al. 2013), disclosing the enormous plasticity of antigen recognition by TCR γδ. So far, there is no evidence of recognition of mycobacterial lipid antigens by γδ cells. Recent studies reported the identification of two mycobacterial proteins, namely of 1-deoxy-D-xylulose 5-phosphate synthase 2 and Rv2272 protein, which activated γδ T cells from pulmonary TB patients (Xi et al. 2013). Responding γδ cells secreted IFN-γ and monocyte chemoattractant protein 1 implicating an important role of these cells in protection and stimulation of monocyte chemotaxis toward the site of infection.
Role of γδ Cells in TB
In TB patients many changes in γδ cell populations have been detected for many years. However, molecular mechanisms and implications in protection and disease progression remain poorly characterized. Only in the recent past have novel mechanisms begun to be carefully investigated. In patients with active TB it was observed a decrease of circulating Vγ9Vδ2 cells and in particular of central memory cells and of effector memory and terminally differentiated cells, which exert immediate effector functions (Meraviglia et al. 2010). These changes reversed after antimycobacterial therapy, suggesting an indirect correlation with antigen burden. In severe TB patients, an increase of γδ cells with enhanced interleukin-10 production was also described (Pinheiro et al. 2012), indicating changes associated with disease progression. This plasticity in T-cell function was confirmed in another study conducted in acute TB patients, in which a progressive reduction of the cytolytic activity of αβ cells was described. However, in the same patients, γδ cells remained perforin+ and capable of killing intracellular bacteria (De La Barrera et al. 2003).
The implication of γδ cells in the immune response within MTB-infected lungs is supported by a series of investigations. Vγ9Vδ2 cells accumulate within lung granulomas (Huang et al. 2012), and in a macaque model of BCG and MTB infection they rapidly expanded upon second exposure to mycobacteria (Shen et al. 2002). This behavior resembles that of adaptive immunity cells and suggests that Vγ9Vδ2 cells may contribute to adaptive immunity to mycobacterial infections. In pleural effusions of TB patients, two populations of γδ cells were identified as TCRlow Vδ2+ and TCRhigh Vδ1+ (Yokobori et al. 2009). The Vδ2 cells were CD45RA+/−CCR7+CXCR3+, released large amounts of IFN-γ, and expressed the CD107a marker, indicating a status of activated cells within the tissue.
Finally, in a mouse model of lung infection with MTB, γδ cells were instrumental to activate DCs in the lung and facilitate subsequent priming of lung CD8 cytotoxic T cells (Caccamo et al. 2006), thus representing important protagonists of the early response during infection.
From <http://perspectivesinmedicine.cshlp.org/content/4/9/a018473.full>