…not so tightly regulated we also have cortisol creating (demand driven) gluconeogenesis when in ketosis or in a fasted state or under stress and was glad you mentioned we have glucagon a cells in our stomach (gastric glucagon).
Sodium and potassium balances are also important even if your not on a ketogenic diet?
Being that sodium does not occur in nature?
Footnotes:
[1] “…Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is ²³Na. The free metal does not occur in nature, and must be prepared from compounds. …” Wikipedia
[2] “…Electrolyte imbalance is markedly present in patients with uncontrolled blood sugars therefore serum electrolytes should be routinely measured in patients with type 2 diabetes mellitus. Serum fasting blood glucose can be used as a predictor for electrolytes. …” …More
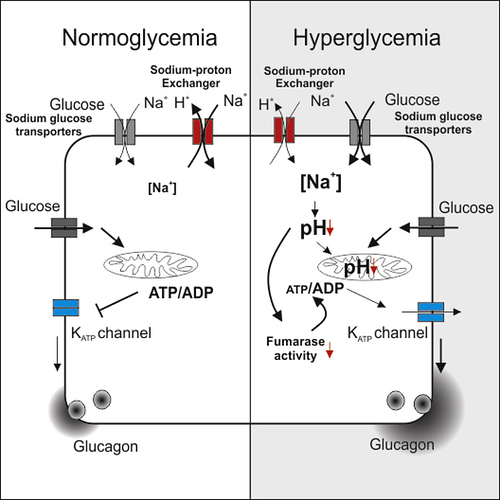
[3] Dysregulation of Glucagon Secretion by Hyperglycemia-Induced Sodium-Dependent Reduction of ATP Production:
“…Type 2 diabetes (T2D) results from a combination of insufficient insulin secretion and defective glucagon secretion and culminates in hyperglycemia (Unger and Orci, 2010). T2D affects every cell of the body, which explains the broad range of complications, including accelerated cardiac and renal failure (Forbes and Cooper, 2013). Dysregulated glucagon secretion in T2D manifests as over-secretion under hyperglycemic conditions but insufficient release under hypoglycemic conditions (Dunning et al., 2005, Rorsman et al., 2014). If not alleviated, hypoglycemia results in glucose deficiency in the brain, coma, and ultimately death. In normal situations, hypoglycemia triggers a counter-regulatory response in the α cells (stimulation of glucagon release with resultant increase in hepatic glucose production), but this does not occur in many type 1 diabetes (T1D) and some T2D patients (Cryer, 1998). Patients with T1D experience on average two episodes of symptomatic hypoglycemia every week (Frier, 2009), and it has been estimated that up to 10% of these patients die of iatrogenic hypoglycemia (Skrivarhaug et al., 2006). Therefore, hypoglycemia has been referred to as the limiting factor in diabetes therapy (Cryer, 2002).
Why counter-regulation fails in diabetic patients is not known, but, interestingly, inhibition of mitochondrial ATP production, or pharmacological activation of KATP channels using diazoxide, recapitulates the dysregulation of glucagon secretion (Zhang et al., 2013). Collectively, these observations suggest that the glucagon secretion defect in diabetic patients is a consequence of disturbed mitochondrial metabolism, but the underlying mechanisms remain obscure.
β cell-specific ablation of the gene encoding the Krebs cycle enzyme fumarase in mice (designated Fh1βKO) results in progressive diabetes. Fh1βKO mice are born normoglycemic and remain so for approximately 8 weeks. Thereafter, they develop hyperglycemia due to loss of glucose-induced insulin secretion (Adam et al., 2017). Here we show that the age-dependent loss of insulin secretion is paralleled by dysregulation of glucagon secretion similar to that in T2D. Similar defects in glucagon secretion develop in other diabetic models: transgenic mice that express a human neonatal diabetes mutation (Kir6.2-V59M) specifically in β cells (Brereton et al., 2014) and Goto-Kakizaki (GK) rats, a model of polygenic diabetes (Granhall et al., 2006).
We have explored the mechanism underlying hyperglycemia-induced dysregulation of glucagon secretion and our data highlight a critical role for Na±glucose co-transport (SGLT)-mediated Na+ uptake and intracellular acidification. We propose that this concept may be extended to other cell types and explain part of the spectrum of diabetes-associated complications. …”
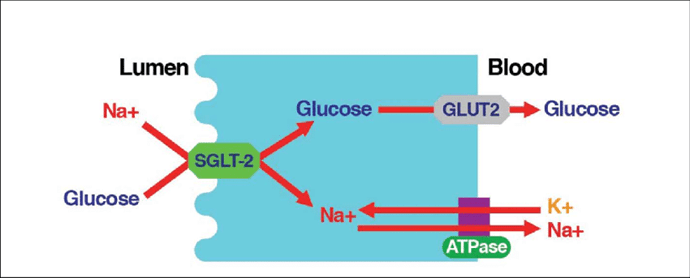
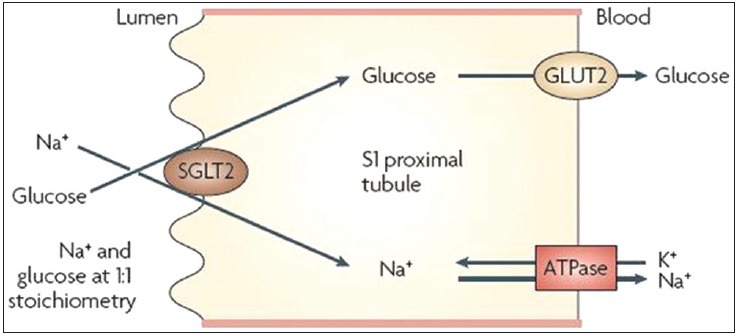
[4] “…INTRODUCTION. Sodium - glucose cotransporter (SGLT) activity mediates apical sodium and glucose transport across cell membranes. Co-transport is driven by active sodium extrusion by the basolateral sodium/potassium-ATPase, thus facilitating glucose uptake against an intracellular up-hill gradient. …” …More>
image link
image link
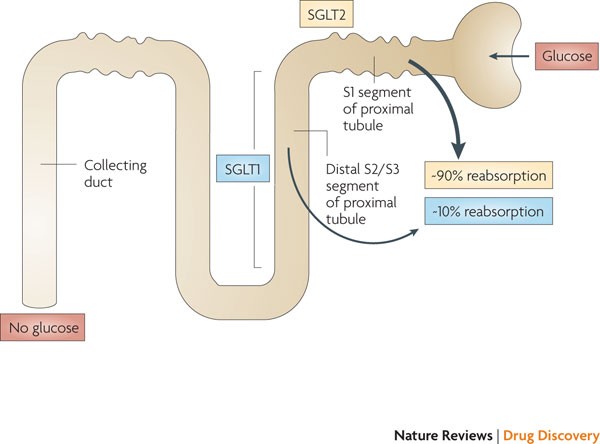 image link
image link
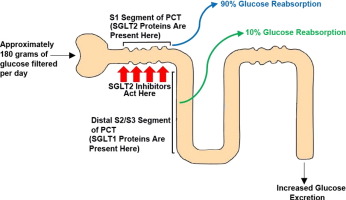 image link
image link
[5] “…Sodium never occurs as a free element in nature. It is much too active. It always occurs as part of a compound. The most common source of sodium in the Earth is halite. Halite is nearly pure sodium chloride (NaCl). It is also called rock salt. Halite can be found in underground deposits similar to coal mines. Those deposits were formed when ancient oceans evaporated (dried up), leaving sodium chloride behind. Earth movements eventually buried those deposits. Now they can be mined to remove the sodium chloride. …” “…People sometimes talk about the amount of “sodium” in their diet. Or they may refer to the amount of “salt” in their diet. The two terms are similar, but not exactly alike. In the body, sodium occurs most often as sodium chloride. A common name for sodium chloride is salt. The Committee on Dietary Allowance of the U.S. Food and Nutrition Board recommends that a person take in about 1,100 to 3,300 milligrams of sodium per day. The human body actually needs only about 500 milligrams of sodium. Studies show that the average American takes in about 2,300 to 6,900 milligrams of sodium per day. This high level of sodium intake troubles many health experts. Too much sodium can affect the body’s ability to digest fats, for example. The most serious problem, however, may be hypertension. Hypertension is another name for “high blood pressure.” A person with high blood pressure may be at risk for stroke, heart attack, or other serious health problems. Sodium is also involved in sending nerve messages to and from cells. These impulses control the way muscles move. Again, an excess or lack of sodium can result in abnormal nerve and muscle behavior. Sodium is also needed to control the digestion of foods in the stomach and intestines. …” …More
[6] “…Salt is composed of two minerals sodium (Na) and chloride (Cl). Table salt (NaCl) contains about 40% sodium and 60% chloride. One teaspoon of salt contains about 2,300 mg of sodium. …” …More
[7] “…Potassium is a chemical element with the symbol K and atomic number 19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. …” Wikipedia
[8] “…In short, aim to consume 3,500–4,700 mg of this mineral per day from foods. People who need more potassium should aim towards the higher end. Summary: A healthy adult should aim to consume 3,500–4,700 mg of potassium daily from foods. …” …More