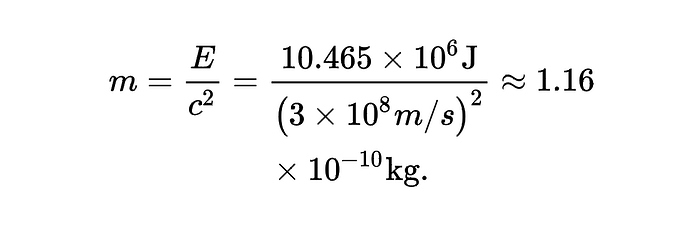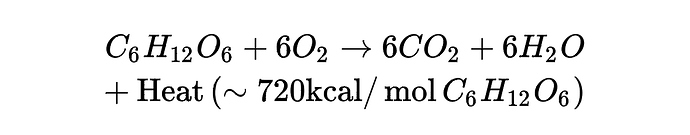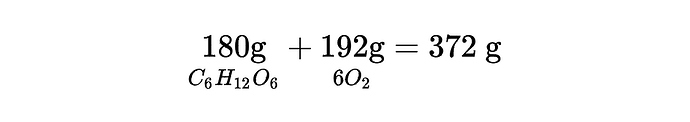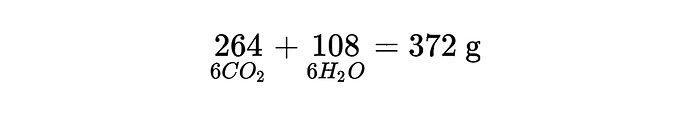A Calorie is Not A Calorie - A Discussion of Thermodynamics
Stokies and CICO die/blow hards
A Calorie Is Still A Calorie - Why Keto Does Not Work :confounded:
Good find.
There are links to similar studies on that page…
Here’s one:
Serious analytical inconsistencies challenge the validity of the energy balance theory
It is concluded, according to the aforementioned model, that weight fluctuations are ultimately dependent on the difference between daily food mass intake and daily mass loss (e.g., excretion of macronutrient oxidation products) and not on energy imbalance. In effect, it is shown that assuming otherwise may caused unintended weight gain.
Thanks @OldDog A plausible alternative to the Energy Balance Model. Excellent food for thought. 
4. Conclusion
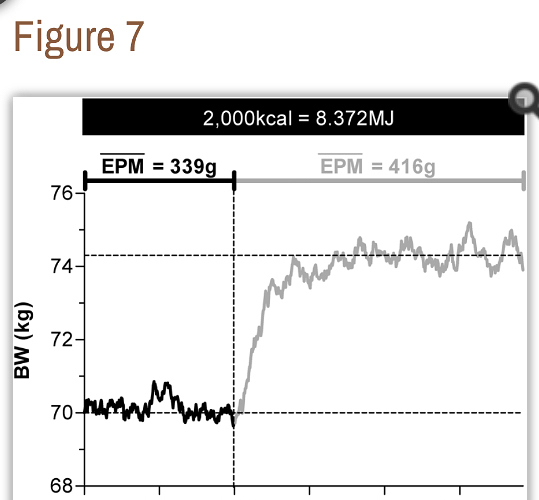
In conclusion, the food property that increases body weight is its mass and not its Calories. The physiological activity that decreases body weight is the excretion of food oxidation byproducts and not heat dissipation. Daily weight fluctuations are thus dependent on the difference between daily mass intake and daily mass excretion indicating that the conservation law that describes body weight dynamics is the Law of Conservation of Mass and not the First Law of Thermodynamics. According to the latter Law, in a closed (Figure 1 A) or open (Figure 1 B) systems, a positive or negative energy balance is not always followed by a similar sign mass change as required by the EBT. This theory is therefore not a corollary of First Law of Thermodynamics; assuming otherwise may have unintended consequences (Figure 7).
“A calorie is a calorie” violates the second law of thermodynamics.
So what? This is not even the argument. Who do you see saying that a “calorie is a calorie,” per se? You don’t see anybody on this forum saying that. What if we were talking about insulin levels, i.e. “IUID” - insulin up, insulin down. There are plenty of people outside this forum who deny the carbohydrate-insulin hypothesis. IUID only tells us so much, and many logical errors can be made by pretending that its application will always be in certain ways. IUID is no different than CICO - it’s valuable to understand what is happening, but it has its limitations and it’s silly to argue against things that are not there.
On this forum, you really don’t see false claims about insulin levels or the direction of calories (energy flow). In this respect, CICO and IUID are the same. People don’t engage in strawman arguments against insulin levels and their possible effect, here, but they sure do engage in strawman arguments against CICO. Why is that?
Here too, so what? Of course there is an equivalence between weight and mass.  It would be silly to argue against that. Likewise, it would be silly to argue that a given mass of carbs, protein or fat had the same energy value, so of course energy balance does not translate into mass change in the same way. And you don’t see anybody doing that - we all know that a given mass of fat has more energy than the same mass of carbs, for example, and we all know that for equal quantities of energy between fat and carbs, the masses are not the same.
It would be silly to argue against that. Likewise, it would be silly to argue that a given mass of carbs, protein or fat had the same energy value, so of course energy balance does not translate into mass change in the same way. And you don’t see anybody doing that - we all know that a given mass of fat has more energy than the same mass of carbs, for example, and we all know that for equal quantities of energy between fat and carbs, the masses are not the same.
This does not mean that the energy balance is not valid. If there is an energy deficit, and fat stores are being consumed, then mass/weight will be lost. This is not saying that it will necessarily be an equal mass to that of the same energy as expressed in carbs or protein. And again, nobody is saying that it would be. Of course the mass balance directly affects weight.  And of course the energy balance effects weight, just not in the same necessarily linear fashion. Increased energy leading to increased energy expenditure can fully accomodate CICO while not leading to the same weight change that increased mass would. This is very basic stuff.
And of course the energy balance effects weight, just not in the same necessarily linear fashion. Increased energy leading to increased energy expenditure can fully accomodate CICO while not leading to the same weight change that increased mass would. This is very basic stuff.
If you want to see this in action, check out @ctviggen’s posted study - Check out my comment I made at KetoCon2019
Increased calories/energy resulted in both weight/mass gain and increased energy expenditure. Both of these are related to the change in calories. It would be nonsensical to claim otherwise. Thus, it’s not only going to be weight change resulting from a change in the energy balance. To act like the greater linear effect of mass change on weight somehow disproves the effects of energy balance is sophistry.
@ElmosUzi Did you read the two articles? Or are you simply responding to the two previous responses? The first article explains exactly how CICO violates the second law of thermodynamics, and is therefore invalid.
The second article offers an alternative hypothesis to the energy balance model of weight gain/loss and stability and the observation and reasoning for it. If the hypothesis is valid the energy balance model may or may not survive and/or require modification. Observation and experiment will be required to determine that.
That was interesting.
After reading, then that brings into question ‘time’ and ‘velocity’ in which it takes something to occur you might have time restricted eating windows and/or fasting that is not being entered into the equations?
But also mass (solid compounds), energy (photons) and time to entropy? For example take a square inch of body fat off your body (how many fat cells does that contain) measured by the circular radius of body in quadrants and how much time it would take to burn one fat cell or all fat cells synergistically…ect.
Sprocketing derailing system on a ten speed or 12 speed bicycle could be good analogy on energy expenditure and caloric intake:
How much energy does it take to push each sprockets into motion on each gear. The fat gear, the carbohydrate gear, and the protein gear. Then you have speed/velocity and duration of time before entropy occurs which are electrical (thermodynamics), chemical (hormonal) and mechanical (physical exertion/motion).
Then you have resistants and impedance, is your energy expenditure going up hill or down hill (propelled forward/free energy)?
[1] “…In nutrition science, EI represents the heat release upon food oxidation [39] and as such it has no contribution to body mass. Einstein’s energy-mass equation shows, for instance, that 2,500 kcal = 10.465 MJ of heat energy are equivalent to:
Daily accumulation of this amount for 100 years would increase body weight by 0.0000042kg. Food’s Calories have, therefore, no impact on body mass. It is food mass that augments body weight; the absorption of 1g of glucose, protein or fat increases body mass by exactly 1g independent of the substrate’s Calories; a consequence of the Law of Conservation of Mass. The level of daily of food mass intake is, however, influenced by the ever present interplay between the environment and genes and by how food’s intrinsic biochemistry relates to satiety [39].
Macronutrients oxidation byproducts are CO2, water, urea, SO3 and heat [16]. Hence, body weight decreases through the excretion of all byproducts except heat. This is exemplified in glucose oxidation:
as the mass entering the reaction (in g/mol) is
while the mass release after oxidation is only present in the reaction products and not in the dissipated heat
Therefore, body mass decreases as we excrete CO2, water, urea and SO3 but not as consequence of the heat content in the EE [40]. The amount of daily mass excretion is, nonetheless, modulated by neural, gastric and endocrine signaling systems that direct body weight regulation [39]. …”
“… 4. Conclusion:
In conclusion, the food property that increases body weight is its mass and not its Calories. The physiological activity that decreases body weight is the excretion of food oxidation byproducts and not heat dissipation. Daily weight fluctuations are thus dependent on the difference between daily mass intake and daily mass excretion indicating that the conservation law that describes body weight dynamics is the Law of Conservation of Mass and not the First Law of Thermodynamics. According to the latter Law, in a closed (Figure 1 A) or open (Figure 1 B) systems, a positive or negative energy balance is not always followed by a similar sign mass change as required by the EBT. This theory is therefore not a corollary of First Law of Thermodynamics; assuming otherwise may have unintended consequences (Figure 7). …” …More
It’s interesting even mind blowing if we are dealing with mass not thermodynamics?
Thermodynamics works if your dealing with more of an electrical system like cold thermal adaption and iron rich mitochondria.
Mass could be as simple as looking at a plate of food and saying well this much food goes from my foot to my knee, then when lunch comes around this amount of food goes from knee to my hip then when dinner comes around, from the hip to the arm pit… not trying to be precise here but you get the idea? Like the term “…you are what you eat from your head down to your feet?..”
Calculating mass rather than how much heat it takes until combustion of each calorie?
You are what you eat by how much (mass) you eat?
Calories don’t matter only amount (mass)?
That just blows my mind! (if true?)
I have always been a stickler about portion size and my notions seem to be correct?
I think the first mistake is to apply physics to something that is not physics. For instance, the human body isn’t a closed system.
The corollary to this is trying to use this to make some type of equation to describe what’s happening. As soon as you do that, you’re wrong. Take overeating studies, for instance. Talk about all over the map. For one thing, protein almost always causes less weight gain. Going strictly by calories, if you increase protein relative to other nutrients (though I personally am loath to call these “nutrients”), you almost always get it wrong.
Hmmm?
All of it is mathematical? Nutritional science, obesity, diabetes and cancer etc. is all based on physics and math, how could it not? No physics or math = shorter life-spans and death? Chemistry, Cellular Biology, Endocrinology etc. all math?
This’ great. While I’ve switched sides on CICO (not saying it’s perfect by any means) because it’s what finally broke an almost year stall for me I draw a line at “a calorie is a calorie”. There’s no reality when you can claim 500cals from meat or vegetables is the same as 500cals from Cupcakes and Twinkies.
There are essentially two ways to see the world we live in, according to the critical realist philosopher Wilfrid Sellars. While I don’t agree with everything he said (and he said some pretty far-out stuff  ), he does make a good point about what he called the “manifest image” versus the “scientific image.”
), he does make a good point about what he called the “manifest image” versus the “scientific image.”
The manifest image is plants and animals, sunsets, rainbows, emotions, contests, anchovies or not on pizza, etc. The scientific image is things at a quantum level, the various spectrums, the electrochemical operations of a cell, units of charge, packets of energy, etc. To varying degrees, people operate in both those ways.
On the manifest side, we more easily acknowledge the mutability of things. You hate anchovies on pizza, I love them - and things mostly run smoothly. On the scientific side, we try and focus more on what’s necessarily true for all of us. Definitions and conceptualizations become more generally important.
CICO seems to be somewhat of an outlier in that people conceptualize it in so many different ways. How does the discussion really go forward if we cannot even agree on what it actually means?
Some think “it’s too simple;” others think “it’s too complex.” Obviously, in the scientific sense it cannot be both. Or not even one of those. It’s really just two statements, wherefrom logic can take us a little farther, but not much. Yet the arguments usually begin with misconceptions about CICO or are even aimed at other people’s misconceptions about it. Is there anything else like this?
Thermodynamics and Metabolic Advantage of Weight Loss Diets
ABSTRACT
Published reports show that low carbohydrate weight loss diets provide a metabolic advantage, a greater weight loss per calorie consumed compared to isocaloric high carbohydrate diets. These reports have not been refuted but rather largely ignored, presumably because of the apparent violation of the laws of thermodynamics (“a calorie is a calorie”). In this review, we show that there is no such violation of thermodynamic laws. Energy utilization of different diets depends on the chemical pathway taken and a metabolic analysis of the efficiency of different pathways reveals large differences. Likewise, thermogenesis produced by diets of different macronutrient composition varies widely. We present a plausible mechanism that depends on the inefficiency of metabolic cycles and, in particular, protein turnover. A low carbohydrate diet makes demands on protein turnover for gluconeogenesis. From a theoretical point of view, energy balance between two diets is to be expected only if the subjects have the same final physiologic state, and only if all of the changes contributing to the energy, heat, work and chemical effects are known. Most diet experiments do not conform to this ideal. There is no theoretical contradiction in metabolic advantage and no theoretical barrier to accepting reports describing this effect.
ABSTRACT
The aim of this review was to evaluate data regarding potential thermodynamic mechanisms for increased rates of weight loss in subjects consuming diets high in protein and/or low in carbohydrate. Studies that compared weight loss and energy expenditure in adults consuming diets high in protein and/or low in carbohydrate with those in adults consuming diets low in fat were reviewed. In addition, studies that measured the metabolizable energy of proteins, fats, and carbohydrates were reviewed. Diets high in protein and/or low in carbohydrate produced an ≈2.5-kg greater weight loss after 12 wk of treatment. Neither macronutrient-specific differences in the availability of dietary energy nor changes in energy expenditure could explain these differences in weight loss. Thermodynamics dictate that a calorie is a calorie regardless of the macronutrient composition of the diet. Further research on differences in the composition of weight loss and on the influence of satiety on compliance with energy-restricted diets is needed to explain the observed increase in weight loss with diets high in protein and/or low in carbohydrate.
Abstract
…In summary, we propose that, in isocaloric diets of different macronutrient composition, there is variable flux of stored TAG controlled by the kinetic effects of insulin and other hormones. Because the fatty acid-TAG cycle never comes to equilibrium, net gain or loss is possible. The greater weight loss on carbohydrate restricted diets, popularly referred to as metabolic advantage can thus be understood in terms of the principles of nonequilibrium thermodynamics and is a consequence of the dynamic nature of bioenergetics where it is important to consider kinetic as well as thermodynamic variables.
Note, TAG: triacylglycerol (triglycerides)
The perception that “a calorie is a calorie” was refuted by Young et al in 1971 (5). They compared 3 diets that contained the same amount of calories (1800 kcal/d) and protein (115 g/d) but that differed in carbohydrate content (3). After 9 wk on the 30-g, 60-g, and 104-g carbohydrate diets, weight loss was 16.2, 12.8, and 11.9 kg and fat accounted for 95%, 84%, and 75% of the weight loss, respectively. Thus, the authors concluded, “Weight loss, fat loss, and percent of weight loss as fat appeared to be inversely related to the level of carbohydrate in the isocaloric, isoprotein diets.”
5. YoungCM,ScanlanSS,ImHS,LutwakL.Effect of body composition and other parameters in obese young men of carbohydrate level of re- duction diet. Am J Clin Nutr 1971;24:290 – 6.
The point of Prof. Feinman’s article is, I believe, that any metabolic theory that claims that all food energy is fungible fails to account for the entropic loss associated with every chemical reaction, hence the invocation of the Second Law. Prof. Arencibia’s article makes the point that it is the mass of food consumed, not its energy, that leads to weight gain. It’s an interesting point, but I may quarrel with his conculsion after finishing the article.
I would simply add that the caloric energy from burning the food item in a bomb calorimeter is not the same as the metabolic energy derived from metabolising the food. For one thing, since protein is not metabolised under normal circumstances, but is rather broken down into its constituent amino acids and rebuilt into other proteins, its caloric value probably should not be counted in the energy intake/output analysis. (Granted, on a low-carb diet, a small quantity of protein is transformed into glucose each day, which is then metabolised, but that amount is very small.)
It should probably be re-stated in this connexion that a food calorie, which a physicist would call a kilocalorie, is the amount of heat required to raise the temperature of a kilogram of water one degree Celsius—whereas the metabolic currency of the human body is in molecules of adenosine triphosphate (ATP) produced. I have no idea how the energy liberated from reducing ATP to adenosine diphospate (ADP) compares with the energy liberated from combustion in a calorimeter, or how to account for the energy required to metabolise fat or glucose into ATP in the first place. It would be nice if someone were to consider these points.
And just to make the standard energy accounting more complicated, the values of 4, 4, and 9 kcals/g for protein, carbohydrate, and fat are rounded from the actual values. Protein and carbohydrate both, if I remember correctly, contain 3-point-9-something kilocalories of heat energy per gram when burned, and the value for fats depends entirely on which fat, and ranges from something like 6-point-something to a little under 10-something. Add to that the difficulties of obtaining a truly accurate figure for energy expended by the body, and we reach the point of absurdity.
Sigh… Wrong. It does not address CICO. This is the same strawman argument that many have commented about - it’s a logical fallacy. This is misrepresenting what the argument is, pretending about what the science is, to try and make it easier to attack. Very much the textbook definition of a strawman argument.
Same question others have asked - who do you see saying that “a calorie is a calorie”? To be clear, this means “per se” or “as simple as that,” as people have mentioned on various threads of this forum.
Exactly.
The “pretend equivalence” is this, in spades. “Calories in, calories out” - how, logically, do we get from there to pretending that calories cannot differ in their effect? Or that CICO is a statement of “equality”? It’s obvious that it’s not. Nobody with any sense is saying they have to be equal.
You found something from 16 years ago that doesn’t talk about CICO. Meanwhile, just last week on the “Spoon-Fed” thread you were saying
This is not true. This is a strawman argument.
@ElmosUzi Addresses CICO right up front in the very first sentence:
The principle of “a calorie is a calorie,” that weight change in hypocaloric diets is independent of macronutrient composition, is widely held in the popular and technical literature, and is frequently justified by appeal to the laws of thermodynamics.
Just expand the parsimony (a scientific principle) of that sentence to its fulness.
The principle of “a calorie is a calorie” [that would be CICO, ie calorie in calorie out], that weight change in hypocaloric diets [that would be calorie restricted diets, ie CICO] is independent of macronutrient composition [that would be a calorie is a calorie no matter what its source nutrient, ie CICO] is widely held in popular and technical literature [that would be diet plans and nutritional advice to ‘eat less and move more’, ie CICO], and is frequently justified by appeal to the laws of thermodynamics [that would be if energy in is less, the eat less part, than energy out, the move more part, then weight loss occurs because of the first law of thermodynamics, ie CICO].
BTW, CICO is actually 3 equations:
CI = CO means no change in weight
CI > CO means gain in weight
CI < CO means loss in weight
In the real world we can’t totally separate mass and energy in food, On @OldDog’s study (above) about energy balance versus mass change, they’re not mutually exclusive. Sure, ‘mass change’ is the bottom line, but the energy balance is a driver of this, as with the body needing to take energy from fat stores. Mass and energy can’t be entirely separated, i.e. if you don’t take in energy you’ll eventually die. I did have to laugh about the “weight fluctuations are ultimately dependent on the difference between daily food mass intake and daily mass loss.” I’ve seen people complain that CICO “is a tautology,” but here we have to realize that for practical purposes with our weight, it’s the same thing as mass  (even going all the way from sea level to the top of Mt. Everest results in less than a 0.3% change in weight). Food energy has to be somewhat fungible. If it’s not totally so, is that a practical concern for us?
(even going all the way from sea level to the top of Mt. Everest results in less than a 0.3% change in weight). Food energy has to be somewhat fungible. If it’s not totally so, is that a practical concern for us?
Been there… We do know about energy consumption and density at the basic levels where they operate in the human body. The ATP to ADP conversion is where essentially ALL our energy comes from. A mole of ATP thus used under physiological conditions gives us ~7.3 calories. At the level of a cell, we know the energy involved in the oxidation of carbon and hydrogen atoms - for decades we’ve known, to a level of accuracy that would not even swing a day’s energy usage for a person by one calorie. That “mole of ATP” is 240.974 grams, so we’re getting only 3% of a calorie per gram.
Does it really matter if we consider calories at the level of ATP conversion or if we just go with the 3500 cals/lb deal? In the end, energy expenditure and weight/mass change (if any) cover such a huge portion of the total picture. Trying to think of food in terms of eventual ATP conversion is enormously cumbersome, by comparison.
This may involve that inseparability or crossover between energy and mass in food. Even if we are not directly using it for energy, it certainly represents mass.
There is a reluctance in the body to metabolize protein for energy. @ctviggen’s study here Check out my comment I made at KetoCon2019 demonstrates this.
There were 3 different groups of people, fed diets with varying percentages of protein: 6%, 16%, and 27%. All diets were definitely hypercaloric. Considering things from the 3500 cals/lb standpoint, those groups gained 69%, 75%, and 80% of the weight predicted from assuming that all excess calories would go to weight gain.
Nothing earth-shaking, so far, but the figures are decently consistent, especially across diets with varied protein content. The higher the percentage of protein, the higher percentage of energy converted to weight there was - makes sense if we view the body as not wanting to ‘burn’ protein.
What’s remarkable is how completely the weight gain and the increased energy expenditure covered the increased calories. I don’t think such accuracy/completeness would have been expected.
Again, is it really practical to worry about the slight possible variances? Going through the calculations and measurements involved with the rule-of-thumb ‘3500 calories’ thing, for example, it works out exceedingly well for human adipose tissue - the triglyceride mix we store as fat, that our fat tissue averages 87% fat, etc.
Energy expenditure is not actually hard to measure. For the best, most complete results, put people in a direct calorimeter. But even indirects ones have displayed very small error, like ~0.25%, and it’s easy to get only 1% or 2% error.