This is the theory that several brilliant scientists proposed more than half a century ago.
Chronically sub-acute (low but not by ‘current standards’) ascorbic leads to degradation of various ascorbic-dependent tissues, particularly to include arteries.
The pressure of blood from the heartbeat, at the point where the arteries have ‘turns’, gradually wears away (because the arteries are weakened) at the wall right where the turn is. This doesn’t happen everywhere. It happens where there is some degree of bend.
The erosion at the point of the bend ‘exposes’ (sort of) some molecules two of which happen to be high receptors for proline and for lysine.
The body creates a larger amount of Lipoprotein(a) in response to the degradation of the arteries. This is because it works well as a gummy-patch over the weakened area. As this situation continues, it patches over the patches until the patches themselves begin to occlude the artery in that location. (Also: other various crap sticks to that area and the L(a) as well. So it’s like a sticky junk pile, which also attracts calcium and calcifies (stiffens).)
Linus Pauling, a Nobel chemistry expert, realized eons ago that simply adding proline and lysine to the blood in a larger dosage – they are nutrients, amino acids – because they had a stronger pull than the receptors, would cause the patch material to bind one molecule at a time to the aminos and carried off.
However you should not do this unless you are also supplementing with ascorbic in order to strengthen the veins. The patches are there for a reason.
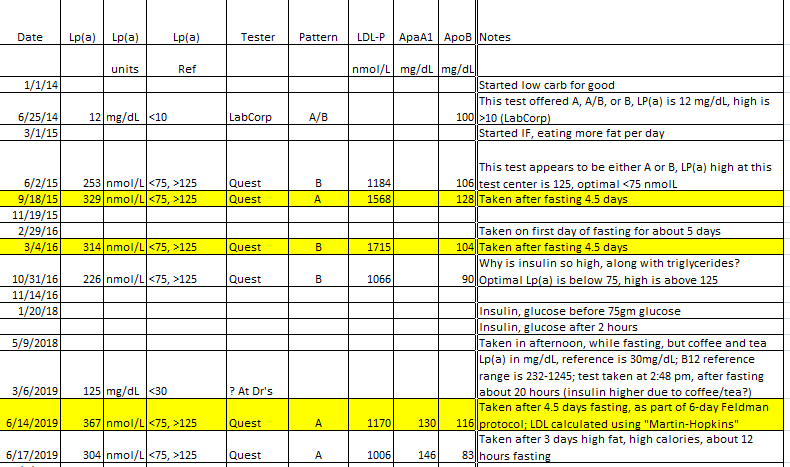
Measuring the L(a) tells you how well your arteries are doing in general (the higher, the worse), but that is more a statement on what the body thinks of their integrity, it only indirectly relates to lesions and patches. It does not tell you whether you have one or more patches which have accumulated to the point of endangering you. There is no way currently to know the state of all arteries in all areas in this regard. We get that by inference, by proxy, from other measures.
The theory is that way back in time when humans were not quite finished baking  a retrovirus affected them (and the primate family), and a few rare other creatures in that region, like a fruit bat. All the rest of the creatures on earth create ascorbic endogenously. Humans have the setup to create it – but at the end point, due to loss of a functional form of the last enzyme (l-gulono-1,4-lactone oxidase) of the biosynthesis pathway, it fails. It is suspected that the early humans who survived this, only did so because they lived in a region where the food supply was extremely high in vitamin C.
a retrovirus affected them (and the primate family), and a few rare other creatures in that region, like a fruit bat. All the rest of the creatures on earth create ascorbic endogenously. Humans have the setup to create it – but at the end point, due to loss of a functional form of the last enzyme (l-gulono-1,4-lactone oxidase) of the biosynthesis pathway, it fails. It is suspected that the early humans who survived this, only did so because they lived in a region where the food supply was extremely high in vitamin C.
Before ascorbic was technically ‘discovered’ they had already decided it was a vitamin and ‘it cured scurvy.’ This created a paradigm in science which is dreadfully unfortunate for humanity and health. At the time it had not yet been synthesized in the lab and was extremely expensive to produce. So even what early research was done with it, was done with incredibly tiny amounts. Which sometimes still had impressive results – but nothing like an appropriate dose would.
Animal nutrition guidelines allot monkeys over 50 times the (comparative) ascorbic that the US RDA says humans need. Guinea Pigs, 40 to over 160 times. A goat’s body can make 15,000 mg on the spot to deal with a “sudden stress.” We are told we “need only a few milligrams of ascorbic” – not even a fraction what our body would naturally make on an easy day, were it not for that enzyme-deficiency. Insufficient ascorbic ensures our glucose handling, hormonal handling, and response to insult, injury and stress are dysfunctional.
Back in time, Diptheria was a big deal and ‘they’ (the science field, funded by interest in a worldwide immunization) were exploring ways of conquering this. Every animal they gave it to shook it off, unless they gave it in such high dose it just killed them outright. Then they discovered the guinea pig. Now this animal is far worse for science than mice and rats due to its lifespan. But it turns out, it cannot endogenously produce ascorbic either. Finally they had a lab rat that they could give the illness to, and then test drugs against. That’s where GP’s became the infamous lab animal, to the point that it’s even seeped into the public lingo where ‘guinea pig’ means ‘experimental test subject’.
So the body, although it gets a tiny amount from food, is not meant to get more than tiny amounts from it. We are supposed to create it internally in whatever dose we need. Ascorbic is not merely a vitamin to prevent scurvy. It does so many things in the body it would absolutely boggle your mind. With the exception of scurvy (including the sub-acute levels common now), everything it does is basically a “support” role. One study on olympic wrestlers showed it reduced cortisol by 20%. It recycles Glutathione and Vitamin E. It does all kinds of things – like I said, the list seems endless, and would be longer, much longer, were it a patentable substance. If you are not aware, ascorbic has seen more bias in every possible way in the science field than any other substance.
As an aside, Szent-Gyorgyi won the Nobel prize for discovering a primary role – more info here:
Ascorbic, like magnesium, has an osmotic effect in the intestines. If you take more than you can absorb at one time, it will pull water in and flush. Ascorbic acid absorbs about 16%. Sodium or Potassium or Magnesium Ascorbate in the low 20’s. Of course, if you are very ill, your body may well use the ascorbate before it even has a chance to reach the end of your intestines, in which case you can take dramatically higher doses. Cathcart did early research on this. You can find more about the flush and dosages here:
The best form is liposomal. Because this is encapsulated inside fats, it is pulled in through the peyer’s patches in the upper intestine, and sent to the liver. This drastically increases the amount one can take at once. The second-best form is a “micro-emulsified” form which is partially liposomal. You can make this at home in quantity using ascorbic acid, sunflower lecithin, and a sonicator, all available online.
For anybody with real interest in this, these links are to an old blog of mine (no ads), and this is the very long but “how we got to current point” introduction to ascorbic:
For those who have interest in creating the micro-emulsified/partly-liposomal version at home (for expense/quantity options), there is a page here, but you should read the introduction page linked above first so it makes more sense:
Trivia: in 2001 I was hospitalized for my lungs due to chronic infections that turned out to be (I later learned) a grain-proteins reaction. During that I was assigned a cardiologist due to a moment of very high stress, pain, and crazy heart rate for a few seconds, and that’s the guy who wrote me a prescription to The Protein Power Life Plan book that eventually (much later) I followed and it saved my life. At that time, allegedly I had crazy high “bad cholesterol” though unfortunately I do not recall details (I wasn’t even given them actually, just the reference from the doc).
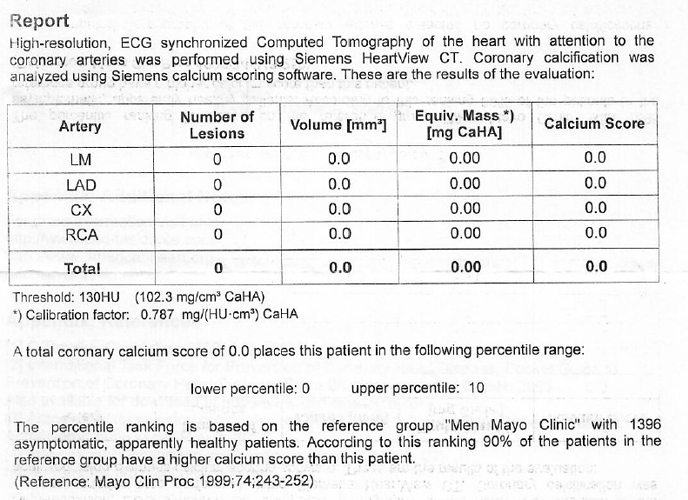
About a year prior to heart surgery I did some of the “Pauling Protocol” which I will put below, for clearing out the arteries. I only did it off and on. I had a lot of other stuff going on frankly (birth defect issue with the valve, in a terrible state). When I got to the surgery, the surgical team told me I had the cleanest veins they’d seen on an adult in eons and even better than many young people. I had zero bypasses as a result. The surgery took half the normal time and I was a day ahead of ideal goal recovery for the next two weeks until I left the hospital for a couple weeks at a care center (my pleading to the doc to assign me this so insurance would cover it) before going home.
I can’t say that protocol helped but something certainly did.
Vitamin C is the main component of the three ingredients in Linus Pauling’s patented preventive cure for Lp(a) related heart disease, the other two being the amino acid lysine and nicotinic acid (a form of Vitamin B3).
Othomolecular protocols:
- Take Vitamin C as ascorbic acid or sodium ascorbate up to bowel tolerance (3 to 18 g) daily.
- Take Lysine, 2 to 3g daily for prevention and from 3 to 6 g daily for the greatest therapeutic benefit.
- Take Proline from 250 mg to 2000 mg daily. (This added factor may lower elevated Lp(a) within 6 to 14 months.)
- Follow Pauling’s general heart and cardiovascular recommendations provided in his book How to Live Longer and Feel Better. Linus Pauling’s Basic Vitamin Program: Vitamin E – 800 to 3200 IU, Vitamin A - 20,000 to 40,000 IU. Super B-Complex, esp. Vitamins B6 and B3
- Supplement Coenzyme Q10 (100 - 300 mg) (High vitamin C and several vitamins will help stimulate your own synthesis of CoQ10 which is vital for proper heart function.)
- Supplement the mineral Magnesium (300 to 1500 mg) and avoid Manganese (No more than 2 mg. USDA researchers report that elevated manganese, more than 20 mg daily, competes with magnesium uptake in the heart causing irregular heart beats.)
- Supplement the amino acids Taurine,Arginine and Carnitine (I to 3 g).
- Avoid refined carbohydrates, especially sugars which crowd out the similar vitamin C molecule in cells.
- Avoid supplemental calcium.
- Add a good mineral/ multivitamin - to cover all possible nutritional needs.
Edited to add: you need a lot MORE vitamin C if you are eating badly. And: L(a) “covers for” lack of ascorbic to a small degree which if you are eating well, is plenty to keep you alive. Lastly: as noted, the vast majority of what ascorbic does is behind the scenes, see the page on Szent-Gyorgyi. Ascorbic is like “inner sunshine.” You can live through cloudy and rainy days and never know the difference. But ascorbic tends to make everything better. We live just fine without it making things better, but when we are faced with almost any kind of assault, it helps a great deal. Most doctors and public only think of it as a prevents-scurvy and anti-oxidant. (Actually it’s also a pro-oxidant. See the Szent-Georgi ref.) It is vastly more.
Some notes on the excellent book by Dr. Thomas Levy about ascorbic which goes through the research that has been done so far for what it helps/does.

 a retrovirus affected them (and the primate family), and a few rare other creatures in that region, like a fruit bat. All the rest of the creatures on earth create ascorbic endogenously. Humans have the setup to create it – but at the end point, due to loss of a functional form of the last enzyme (l-gulono-1,4-lactone oxidase) of the biosynthesis pathway, it fails. It is suspected that the early humans who survived this, only did so because they lived in a region where the food supply was extremely high in vitamin C.
a retrovirus affected them (and the primate family), and a few rare other creatures in that region, like a fruit bat. All the rest of the creatures on earth create ascorbic endogenously. Humans have the setup to create it – but at the end point, due to loss of a functional form of the last enzyme (l-gulono-1,4-lactone oxidase) of the biosynthesis pathway, it fails. It is suspected that the early humans who survived this, only did so because they lived in a region where the food supply was extremely high in vitamin C.