Insulin is not Needed for Glucose Uptake and Utilization in Humans
Contrary to popular belief supported by the leading physiology and biochemistry textbooks, there is sufficient population of glucose transporters in all cell membranes at all times to ensure enough glucose uptake to satisfy the cell’s respiration, even in the absence of insulin [21]. Insulin can and does increase the number of these transporters in some cells but glucose uptake is never truly insulin dependent. Even under conditions of extreme ketoacidosis there is no significant membrane barrier to glucose uptake – the block occurs “lower down” in the metabolic pathway where the excess of ketones competitively blocks the metabolites of glucose entering the citric acid cycle. Thus, insulin is not needed for glucose uptake and utilization in man [21]. In fact, the process appears to be general for all polar (water-soluble) substrates, as transporters are the mechanism by which they are transported across the highly non-polar (lipid) cell membranes. When insulin is administered to people with diabetes who are fasting, blood glucose concentrations falls. It is generally assumed that this is because insulin increases glucose uptake into tissues. However, this is not the case and is just another metabolic legend arising from in vitro rat data. It has been shown that insulin at concentrations that are within the normal physiological range lowers blood glucose through inhibiting hepatic glucose production [21].
Insulin Not Needed for Glucose Uptake and Utilization in Humans
Interesting paper @amwassil and totally not what I pictured happening but actually makes really good sense.
Ok so I’m reading through this very carefully, as a personal trainer and also as an athlete that’s been fat adapted for years…I think this paper is saying that amino acids are a useable and desirable fuel source. This is contrary to my understanding that “protein” (ie amino acids) is not a good energy substrate…I’m just looking for clarification 
I have cited this authors paper on the forum countless times but the author has this to say:
image link”…Thus, both muscle fat[2] and carbohydrate burn in an amino acid flame. …”
That is very thought provoking and much deeper than the statement below, because you will often times hear or see the opposite:
”…fat burns in a carbohydrate flame…”
Without insulin we would simply turn into bag of sugar and die[1] with or without carbohydrates?
What I have noticed is that genuine Type 2 diabetics seem to fair well on a long-term low carbohydrate, high fat diet than a more metabolically fit person, and can tolerate 20 grams of carbs or less very long term or what almost seems indefinite amount of time being the human body is literally a sugar factory even without carbohydrates or low carbohydrates? (am I the only one seeing that?)
Footnotes:
[1] ”…This all depends, of course, on the human body’s capacity to survive without any insulin** . …Insulin is the only antidote. Even if you eat nothing, your body metabolizes itself, breaking down fat and muscle into simple sugars. Sweet poison pools in your limbs and you die. …” …More
[2] “…Defects in mitochondrial substrate selection, mediated by inhibition of the pyruvate dehydrogenase complex (PDH), have been proposed to be a major contributor to lipid-induced muscle insulin resistance. To examine this hypothesis, we assessed insulin action in a genetic mouse model of constitutive PDH activation. Surprisingly, we found that preferential glucose oxidation in skeletal muscle in this mouse was accompanied by muscle insulin resistance. Muscle insulin resistance could be attributed to increased glucose oxidation at the expense of reduced fatty acid oxidation, leading to increased intramyocellular lipid accumulation and diacylglycerol- PKC-θ–mediated reductions in proximal insulin signaling. These findings have important clinical implications for novel anti-diabetic therapies currently in development that activate PDH and enhance glucose oxidation in muscle. …” …More
[3] How is pyruvate converted to glucose?
The principal substrates for gluconeogenesis are amino acids (alanine and glutamine) derived from muscle protein, glycerol derived from the triglycerides stored in adipocytes, and lactate from muscle. Liver glycogen is the major body store of carbohydrate for rapid release of glucose via glycogenolysis. …More
[4] What hormone inhibits gluconeogenesis?
Gluconeogenesis is stimulated by the diabetogenic hormones (glucagon, growth hormone, epinephrine, and cortisol). Gluconeogenic substrates include glycerol, lactate, propionate, and certain amino acids (alanine and glutamine). PEP carboxykinase catalyzes the rate-limiting reaction in gluconeogenesis. …More
[5] “…In beta cells, insulin release is stimulated primarily by glucose present in the blood. As circulating glucose levels rise such as after ingesting a meal, insulin is secreted in a dose-dependent fashion. …Metabolism of the glucose produces ATP, which increases the ATP to ADP ratio. …” …More
[6] Metabolic Inflexibility Impairs Insulin Secretion and Results In MODY-like Diabetes
in Triple FoxO-Deficient Mice: Pancreatic b cell failure in type 2 diabetes is associated with functional abnormalities… …Insulin secretion is induced by rising ATP/ADP ratios (Mat- schinsky, 1996).
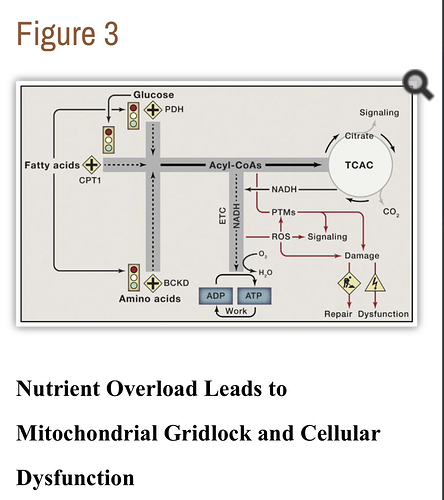
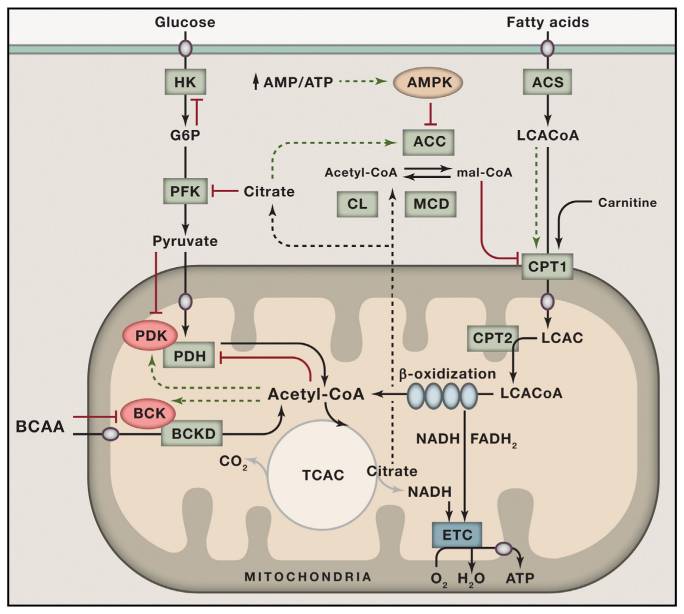
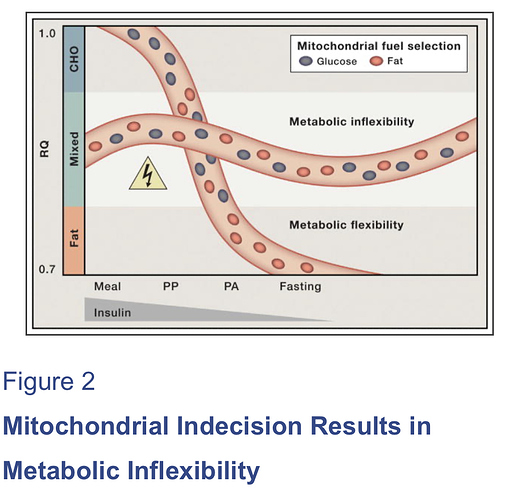
[7] “…In recent years, these two camps have found common ground in the concept of metabolic flexibility, which posits that cells function optimally when they retain their capacity to switch freely between oxidative substrates in response to nutritional and physiological cues. The foregoing network model of metabolic flexibility further suggests that, during conditions of inactivity and low ATP demand, mitochondria function best when acetyl-CoA is produced from one fuel at a time. Robust and decisive shifts in substrate choice are predicted to limit mitochondrial congestion and damaging molecular collisions while also producing strong and clearly interpretable metabolic signals that guide efficient nutrient partitioning to maintain energy homeostasis. This model provides an explanatory context for viewing the severe costs of excessive food consumption and the benefits of habitual physical activity and lifelong caloric restriction. …” …More
[8] “…A new pathway that triggers regeneration of beta cells in the pancreas, a key development that may aid in the development of diabetes treatments, has been discovered by scientists. … Insulin, a hormone that regulates blood sugar, is made in pancreatic beta cells. …” …More
How much collagen in everyday foods?
Why Don't we Get Hungry During Extended Fasting?