I wanted ask anyone who is interested, what would they make of this?
What can be derived from this?
What is your take or opinion?
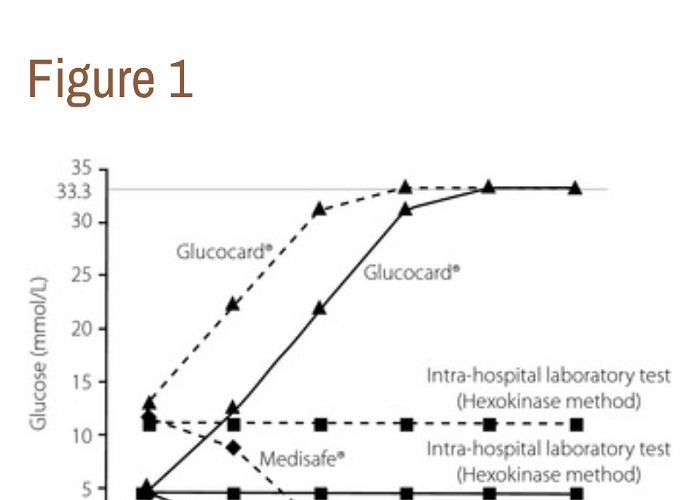
“…A 71‐year‐old woman with advanced esophageal cancer and diabetes presented to the Department of Metabolic Medicine, Osaka University Hospital, Suita, Japan, for glycemic control in November 2012. At the first visit, her glycosylated hemoglobin level was 8.8%. SMBG was carried out using the Medisafe® meter (TERUMO Corp., Tokyo, Japan) to determine the dosage of regular insulin before every meal. One day, she was given a high ascorbic acid dose (50 g) in another hospital. Her blood ascorbic acid concentration just after administration was 342 μg/mL (normal range 4.7–17.8 μg/mL), and her blood glucose level determined with the Medisafe® meter was below 1.1 mmol/L, which was ‘low’. Simultaneously, glucose levels were measured using the Glucocard®meter (ARKRAY Inc., Kyoto, Japan). According to the Glucocard® meter, her blood glucose level was above 33.3 mmol/L, which was “high.” She had neither hypoglycemic nor hyperglycemic symptoms. The fasting blood glucose level (9.3 mmol/L) determined with the Medisafe® meter 2 days after administration was close to the level (9.2 mmol/L) determined using the intra‐hospital laboratory test based on the hexokinase method (Quickauto‐Neo GLU‐HK; SHINO‐TEST Corp., Tokyo, Japan) and using an automated analyzer (JCA‐BM6050; JEOL Ltd., Tokyo, Japan). Then, the treatment with intravenous high‐dose ascorbic acid was discontinued with her consent. We prepared blood samples that contained 4.4 and 11.1 mmol/L glucose, and 0–500 μg/mL ascorbic acid. The glucose levels increased in accordance with the increased ascorbic acid concentrations using the Glucocard®meter, whereas they decreased using the Medisafe® meter. However, the glucose levels measured using the intrahospital laboratory test did not change in response to any ascorbic acid concentration (Figure 1).
“…The Medisafe® meter is based on the colorimetric method, which is applied to several types of Accu‐Chek® meters (Roche Diagnostics, Mannheim, Germany), whereas the Glucocard® meter is based on the electrochemical method, which is applied to many glucose meters, including the One Touch Ultra® (LifeScan Inc., Milpitas, CA, USA), FreeStyle® (Abbott Diabetes Care, Alamdea, CA, USA) and Contour® (Bayer Healthcare, Tarrytown, NY, USA) meters. As hydrogen peroxide is consumed by ascorbic acid, less hydrogen peroxide is available to react with the dye on the test strips, leading to falsely lower glucose readings by the Medisafe® meter. In contrast, ascorbic acid is oxidized at the electrode surface, and more electrons and more current are produced, leading to falsely higher glucose readings by the Glucocard®meter2. The critical glucose measurement interferences by high‐dose ascorbic acid could cause inappropriate insulin therapy or glucose administration, resulting in serious hypoglycemia or hyperglycemia. We need to understand that this type of interference occurs at the bedside; therefore, blood glucose levels just after intravenous administration of high‐dose ascorbic acid should be measured using the hexokinase method, not with these SMBG meters. …” …More