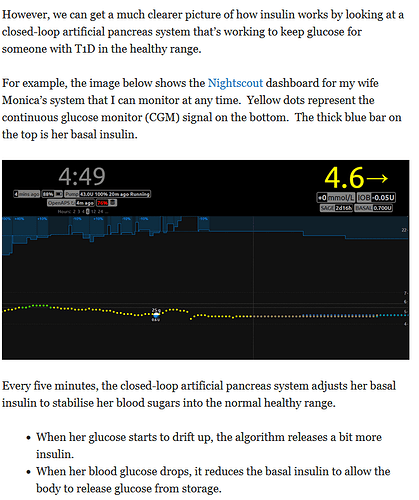
Where do I start.
Well the first is to establish my priors. Marty blocked me on FB sometime back because I poked holes in his favourite “satiety per calorie” theory, by suggesting that in his list of the most satiating and least satiating magic food he has missed fasting which can in context be quite satiating. Do I think he’s a muppet … well yeah, but that doesn’t mean he might not have some good ideas so let’s see now shall we?
If his primary thesis that humans eat calories until they meet their requirement for micro nutrients were true, then a multivitamin would be the greatest diet pill the world has seen. Since Weight Watchers (which advises clients to take multivitamin pills) have such a poor track record of success, and there is therefore a market for businesses like Marty’s that teach people hacks to get them to eat less then you would have to infer that the mere existence of his business suggests that his hypothesis is incorrect.
Now, I’m not sure why we should expect a civil engineer to know more than a biochemist (or the entire literature) about the triggers of insulin secretion, but lets ventilate his theory that the role of insulin is not to regulate the fuel partitioning between the fed/fasted state, but rather to … checks notes … hold in fuel in fat cells so your liver doesn’t disintegrate, or some such. So if that is true then clearly the fat cell must have some mechanism to dial up more insulin from the pancreas.
The direct triggers of pancreatic insulin secretion have been experimentally shown to be glucose, mannose (at 6x the concentration of glucose), and to a lesser degree arginine and leucine (via leucine hypoglycemia studies).
Fat cells can not dial up insulin by making any of these. But it is possible for a fat cell that is releasing fatty acids from stored triglycerides to cause more glucose stimulated insulin to be made. One of the byproducts of lipolysis, mono-acyl-glycerol potentiates glucose stimulated insulin secretion. But they can’t do it without glucose and the only role they have is to make the resulting release of insulin in response to glucose greater. This is at most a second order effect so the hypothesis is unlikely to be true.
The problem with inferring causation from an apparent association between two observations (people who are fat, and people who have high insulin) is that there are 5 possible ground truths that could all lead to the same outcome;
- A could cause B
- B could cause A
- C could cause both A and B
- A could cause C which causes B which causes D which causes A again
- A and B could be independent but you’ve chosen such a small sample size that your experiment was insufficiently powered.
Marty’s claim is that B causes A - ie: ↑Fat ⇒ ↑Insulin. In fact his claim goes further that A can not cause B, ie: ↑Insulin ⇏ ↑Fat
The literature, and this biochemist, claim that at the very least A causes B; ie: ↑Insulin ⇒ ↑Fat, but more likely A causes C caused B causes D causes A again ie: ↑Insulin ⇒ ↓fatty acid oxidation ⇒ ↑lipid buffering ⇒ ↓glucose transport ⇒ ↑Insulin .
In this case there is a way to disprove Marty’s claim that insulin doesn’t cause fat through a simple experiment. If we can show in the absence of obesity, that there is a linear dose relationship between insulin and fatty deposition then his claim is not even wrong.
This study [https://www.ahajournals.org/doi/pdf/10.1161/01.res.9.1.39] made 16 dogs chemically insulin dependent (by killing their beta cells) and then give the dogs the insulin they now must have … but do it in just one femoral artery. That way the circulation takes that insulin down one leg, back to the heart, to the lungs, back to the heart and then to the other leg. The further tissue is from the site of injection the lower the concentration. So if you see a differential increase in fatty deposition in the leg that was given the insulin vs the leg that wasn’t (and had therefore a lower exposure to insulin), then you can conclude that insulin causes an increase in fat deposition. And that is exactly what was observed.
AFAIK Marty has 2 type 1 diabetics in his immediate family, so I’m surprised that he doesn’t know that insulin dependent diabetics have to rotate the location they inject insulin otherwise they get unsightly fatty deposits around the injection site. He might be confused because insulin is injected into fat tissue that the appearance of fatty deposition at the injection site is caused by insulin locking in fat in fat cells. If you expose any human cells to insulin they buffer fatty acids instead of burning them. Those dogs didn’t just get more fat in fat cells they got more fat buffered in muscle cells, in epithelial cells of the blood vessels, and fat cells, and every cell exposed to the higher concentration of insulin.
I’m not even going to give that strawman of fat being a free food the respect it doesn’t deserve … as my favourite author Robert Heinlein said “TANSTAFL”. However fat to satiety is a claim made by Phinney and Volek and THAT has been thoroughly tested experimentally many times. It neither a priori increases body fat, nor insulin resistance - evidently (as in as shown by experimental evidence) usually it is quite the opposite.
I think we’ve covered why elevated insulin and insulin resistance results from fat gain is a fatuous statement … but let me explain why insulin and insulin resistance are actually caused by fat gain but not in the tissue Marty thinks, or the mechanism he thinks he understands.
Let’s start with insulin for the sake of simplicity. How much insulin you make is dependent on how well you can clear glucose from circulation into your cells, which is the definition of insulin resistance. The more insulin you need to make for the same clearance of glucose, the more insulin resistant you are. But insulins role is not just to transport glucose into cells, it also inhibits transport of fatty acids into the mitochondria.
The TLDR version of that is that insulin signaling promotes the transcription of and activates by dephosphorylating the enzyme acetyl-CoA carboxylase which is the first committed step of and rate limit of de-novo lipogenesis. One intermediate product or making new fats from old citrate, malonyl-CoA, allosterically inhibits carnitine palmitoyl transferase required to transport long chain fatty acids across the mitochondrial membranes … so not only are you making new lipids when insulin is elevated, but you are not burning them either, so they buffer in lipid droplets in the cell, and the cell stops importing new lipids, so they buffer in circulation, and they buffer ultimately in the final buffer … fat cells.
There are only 2 ways to get fatty acids out of the body, respiration and lactation. All the other fuels (glucose, lactate, amino acids, ketones, citrate) are water soluble and can be filtered out by the kidneys. But if you aren’t a lactating woman, then the only way to get rid of fatty acids is to get them into the mitochondria and turn them into CO₂ and H₂0. And as long as insulin is elevated you are inhibited from doing that so they buffer in lipid droplets. Lipid droplets are micells made of a ball of lipids with a surface covered in phospholipids and proteins.
One of those proteins is a SNARE protein that is used to allow the micel to fuse with the outer mitochondrial membrane to deliver fuel to the mitochondria once insulin drops. And here is how that fat build up, in all tissue (not just adipocytes) loops around and causes insulin to go up. The glucose transporter also uses the same SNARE protein to fuse with the plasma membrane to import glucose into the cell. If you have more and larger lipid droplets, then GLUT4 gets fewer SNARE proteins and has a harder trouble fusing with the plasma membrane to let glucose into the cell.
That means that you clear glucose slower, which means your pancreas makes more insulin … and the cycle continues. A causes C causes B causes D causes A again. People who claim “B causes A” can be the only mechanism are over reducing a complex system to the point that they can fit it in a bromide to sell a product, but the statement has lost all usefulness. It’s the biochemical version of “4 legs good, 2 legs bad”