Acetone in the breath is being wasted, so I’m not sure how accurate a measure it would be of ketone production or consumption, quite apart from any issues with the accuracy of the meter itself.
Coconut Oil lowers (not raises) Ketosis Level
Bunny, Thanks for the great answers getting to the question of Why this may be happening. I did included both glucose and glycogen in my thinking, as well, cortisol (which Stan Ekberg often mentions). This was an informative answer and I’ll look for the Duke U physiology book too! I think also there are brain tumor pituitary gland effects on insulin resistance (or at least release)… Taubes mentions this and the fat mice/rats who were doctored to starve.
Paul, Yes, thought of this… the body and biochemistry is all about enzymatic control loops and they really can’t go “out of balance”. That’s why “a lowering” of the breath ketones was of interest to me… it made me conclude that consumption of Coconut (saturated fats + MCT) must have a calling in effect on ketone levels… couldn’t rationalize why, as more available fat in blood serum should imply more ketone creation in the liver BUT as bunny points out, not necessarily… that’s a control loop in liver as well.
Glucagon is the main stimulator of gluconeogenesis and ketogenesis. It doesn’t take much glucose to keep the body running. The standard figure is that no more than a teaspoon (5 mL) of glucose is circulating in the blood at any given time, when the person is healthy (and not overloading his or her system by eating too much sugar or carbohydrate). Hypoglycaemia is not much of a risk in nutritional ketosis or fasting ketosis, since the ketones (acteone and β-hydroxybutyrate, if I’ve got it right) feed the almost all the brain’s needs quite nicely. (And the teaspoon of circulating glucose takes care of the rest. Apparently, just how much glucose the brain really needs is under dispute.) Don’t forget that unethical experiment Cahill’s team did with their fasting subjects, using an insulin clamp to drive down their serum glucose to levels that normally cause coma and death. But because the subjects were deep into fasting ketosis, they were perfectly fine and exhibited no symptoms of hypoglycaemia.
By contrast, insulin is the primary inhibitor of gluconeogenesis and ketogenesis. This makes sense, when we think about it, because if we are eating carbohydrate we want to (a) stop making our own, and (b) get it out of the bloodstream by storing it in adipose tissue as fat (think of bears getting ready to hibernate) and metabolising it in the muscle. When we ingest a quantity of carbohydrate or sugar, preventing hyperglycaemia becomes the challenge, instead of preventing hypoglycaemia.
Bob, Thanks for the reference to the bulletproof coffee differences. In my mind, the fatty acids are different but we know they are convertible. There is likely an application difference throughout the body and Coconut Oil is known high in saturated fats + the MCT. The saturated fats may trigger other cells to absorb more of the total fats in the body including the ketones which I think can be freely carried in the blood.
Not sure I’m following the logic here, unless what you are saying is that the influx of exogenous ketone bodies temporarily halts ketogenesis, since there is no need for more ketone bodies at the moment.
There is a lot to unpack with ketosis, because the various ketone bodies are not just sources of fuel, but also potent epigenetic signals, as we are learning. It would probably be important to know just what exogenous ketone bodies are found in the coconut oil you were using. I just watched a fascinating presentation on the subject over the weekend, and the effects of the different ketone esters and salts are not altogether known. There are also two different chiral isomers of β-hydroxybutyrate (laevo- and dextro-rotatory), and it is not really known whether or how the body handles L-β-hydroxybutyrate; we just know that what the liver produces is D-β-hydroxybutyrate.
Bunny, Do you know more about the somatostatin and catecholamines (epinephrine and norepinephrine) effects described in the paper. Especially the antagonistic effect of catecholamines metabiling glucode stores during periods of stress. And relation to serum blood levels … or more specifically ingestion… My first mention of this effect I forgot the observation that it appeared almost immediate which is unusual with ketone response. But we know, exogenous ketones can be ducted to the liver immediately from the intestine to produce such quick responses.
Paul, Yes! I’m very interested in ketosis with signal transduction, hormonal effect of the ketones and their epigenetic affects signalling autophagy and reverse (anabolic) effects in the cells and body!
Paul, I’ve seen some of Stan Ekberg’s talks on ketones as hormones and someone else… please include a reference to the video you just watched. Thank you!
From the research what is interesting is the time parameters on fasting like a 96 hour fast peak for Somatostatin binding receptors but it seems that beta (insulin) and alpha (glucagon) cells including Somatostatin f cells (d = polypeptide) on the pancreas die (slough off) and regenerate at great speeds almost like how nails grow?
It’s almost as if these type of pancreatic cells are appendages that adjust to diet, they are not hermetic? (dynamic not static) Then due to dietary factors the regeneration pancreatic (stem) cells reduce in number and not enough insulin?
In other words they cannot regenerate fast enough to keep up with the constant bombardment of dietary glucose? They are intended only to deal with endogenous glucose?
[1] ”…The somatostatin concentration increased in fundic and antral mucosa after 24 h and reached its highest value after 96 h of fasting. The number of specific somatostatin binding sites with high and low affinity decreased with the duration of fasting . …” …More
After looking at this if people do not listen to Dr. Fung they are doomed?
Bunny, Fascinating details. I’ve been practicing multi-day fasts (with supplements and coffee during fast) and over a 1 1/2 to 2 year practice I’ve kind of zeroed in on 5 days fasting (120hrs) 2 days feast… but find I get very hungry towards a now routine target end of fast… someone asked why I was looking at Coconut oil adder, and it’s to remove all need for exit to fast based on hunger or metabolic drop-off. The diet adjustment somatostatin f cells to peak at 96 hrs correlates well with known patterns of fasting that Jason Fung et. al. have described. Can you put a reference citation for the book you mentioned that you are excepting? Thank you! Best regards, Mike
Dave, Just a note to you, myself and others interested… after several days of consuming Coconut oil in my coffee I am No Longer observing an breath measured, ketone level, Lowering! I attribute the initial observation to a cephalic or hormonal anticipatory effect which I’ve now become adapted to again. In deference to your disbelief this Was a real effect as far as I can determine.
[1] “…Stem cells: Now, in a manuscript published online in Trends in Endocrinology & Metabolism, scientists from the Diabetes Research Institute at the University of Miami draw categorical conclusions in support of the original theory that progenitors in the pancreas do exist and, moreover, that these stem cells may regenerate in human …” “… “We have demonstrated that there are progenitors in the adult pancreas, not only in mice but in humans, which is a very important clarification, and that those cells can potentially be stimulated through pharmacological means to induce regeneration in patients with type 1 diabetes. That is the ‘Holy Grail’ of what we are trying to achieve here at the DRI,” said Juan Dominguez-Bendala, Ph.D., director of pancreatic stem cell development for translational research and co-author of the paper with Ricardo Pastori, Ph.D., director of molecular biology.
Flawed Techniques Shift the Hypothesis
In the 1980s, researchers logically concluded that the pancreas harbors progenitor cells capable of regenerating endocrine (insulin-producing) cells after an islet was photographed sprouting from an adult pancreatic duct. Over the three decades that followed, dozens of reports further reinforced the idea that a variety of growth factors could stimulate ductal cells to differentiate into all pancreatic cell types, including insulin-producing cells. …” Dec 4, 2018 …” …More
[2] ”…Alloxan (used to bleach milled grain flour to make white bread?) and streptozotocin are toxic glucose analogues that preferentially accumulate in pancreatic beta cells via the GLUT2 glucose transporter. …Owing to its alkylating properties, the latter modifies biological macromolecules, fragments DNA and destroys the beta cells, causing a state of insulin-dependent diabetes. Dec 18, 2007 …” …More
[3] “…Cooked common beans (Phaseolus vulgaris) protect against β-cell damage in streptozotocin- induced diabetic rats. …” …More
[4] “…Streptozotocin or streptozocin (INN, USP) (*STZ ) is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia and Alzheimer’s in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses. …” “… Streptozotocin was originally identified in the late 1950s as an antibiotic.[7] The drug was discovered in a strain of the soil microbe Streptomyces achromogenes by scientists at the drug company Upjohn (now part of Pfizer) in Kalamazoo, Michigan. The soil sample in which the microbe turned up had been taken from Blue Rapids, Kansas, which can therefore be considered the birthplace of streptozotocin. Upjohn filed for patent protection for the drug in August 1958 and U.S. Patent 3,027,300 was granted in March 1962. Recent advancements in understanding the biosynthesis of this natural product have been made by Balskus et al. [8] In short, the authors found the gene cluster responsible for production of Streptozotocin in Streptomyces achromogenes and identified novel function of a non-heme iron enzyme, SznF, which forms the N-N bond in the N-nitrosourea pharmacophore by oxidative rearrangement. In the mid-1960s, streptozotocin was found to be selectively toxic to the beta cells of the pancreatic islets, the cells that normally regulate blood glucose levels by producing the hormone insulin. This suggested the drug’s use as an animal model of diabetes,[9][10] and as a medical treatment for cancers of the beta cells.[11] In the 1960s and 1970s, the National Cancer Institute investigated streptozotocin’s use in cancer chemotherapy. Upjohn filed for FDA approval of streptozotocin as a treatment for pancreatic islet cell cancer in November 1976, and approval was granted in July 1982. The drug was subsequently marketed as Zanosar. Streptozotocin is now long off patent and many generic formulations are available. …More
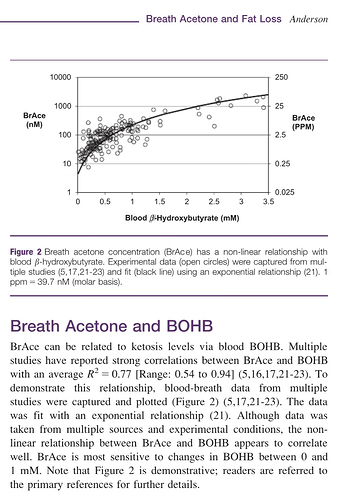
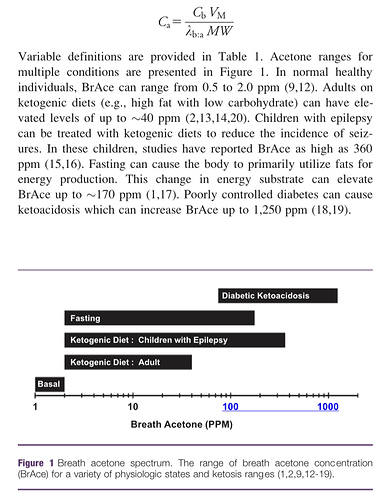
That’s interesting. BrAce is, of course, one break down product of acetoacetate; CO2 is the other. Thus, the concentration of BrAce is directly dependent upon not only the quantity of aceotacetate in the blood, but it’s rate of decay. And I don’t know if that rate is known precisely or not. If known, then BrAce could be used to estimate acetoacetate concentration. The folks at Ketonix make that claim, of course.
One would suspect that the more acetoacetate in the blood the more BrAce would be exhaled. Conversely, the less acetoacetate the less BrAce. So a logical conclusion to falling BrAce would be falling acetoacetate. Acetoacetate also ‘spontaneously’ converts to/from β-hydroxybutyrate. β-hydroxybutyrate does not decay into BrAce. It must first convert back to acetoacetate. So if a lot of acetoacetate converts to β-hydroxybutyrate for example, I would expect a smaller amount of BrAce to show up in the breath.
You could test this easily if you have a blood test device. When your BrAce is falling measure your blood to determine whether β-hydroxybutyrate is increasing. If so, then we could conclude that metabolizing coconut oil results in higher conversion into β-hydroxybutyrate where it and the most of the remaining acetoacetate get oxydized. Now that would be quite an interesting thing to know.
PS: You could also repeat this experiment using pure MCT, dairy butter and cacao butter to see if they do the same thing or something different.
That’s Anderson’s study and I am familiar with it because the folks at Ketonix use it to back up their claim that BrAce correlates directly to β-hydroxybutyrate. I am not aware of any other study that replicates its results. The problem I have with it is that BrAce is a direct product of the breakdown of aceotacetate while β-hydroxybutyrate is a direct product of the conversion of acetoacetate into a more stable molecular structure, which prevents its breakdown into BrAce. It seems to me that you have two different products generated from acetoacetate and if you have more of one you have less of the other. Zero sum, so to say. If you have a fixed amount of acetoacetate, the more that converts to β-hydroxybutyrate the less is left to breakdown into BrAce. Plus, acetoacetate itself gets utilized as fuel directly before it spontaneously breaks down or converts to β-hydroxybutyrate. I’m not saying Anderson is wrong, so much as maybe he measured BrAce and β-hydroxybutyrate while acetoacetate was increasing due to some factor.
What we have here, is a report that ingesting coconut oil apparently results in reduced BrAce. If Anderson is correct, then we should see a corresponding decrease in β-hydroxybutyrate as well. My suggested experiment would determine whether that’s the case or not.
The exact breakdown and conversion of ketones is interesting.
Could it be that when we have all these exogenous fatty acids in circulation that the liver and adrenals just stops pulling it from fat cells when it has too many ketones it is not using for fuel to begin with and then we throw coconut oil and dietary fat on top of that, I can see why people get nowhere with burning body fat for fuel without fasting?
Observing ketones really does not matter when you put any kind of exogenous fats or ketones in your body?
Eating more fat is good if your thin but eating all this fat frame work (forgot to take off the training wheels?) is part of the problem, your body including your brain can only burn so many ketones or fatty acids for fuel, where is it coming from?
Then you have amino acids coming in from protein for fuel and the rest is glucose and mostly glucose for glucose dependent organs and processes?
How long does it take to burn through that stick of butter and steak you ate two days ago before your body goes after it’s own fat?
Then you have the problem of re-esterification (fatty acid turn over pool) of fatty acids where the fat and dietary fat get reabsorbed back into the fat cell and then the rest just floats around in your blood stream? Just like glucose?
Here’s one specifically about BrAce:β-hydroxybutyrate which concurs with Anderson’s study.
The first study is interesting
“…Our results suggest that time-restricted feeding regarding carbohydrates may optimize ketosis from intake of MCT. …” …More
Second study:
”…C8 was about three times more ketogenic than C10 and about six times more ketogenic than C12 under these acute metabolic test conditions, an effect related to the post-dose increase in octanoate in plasma total lipids. …” …More
Third Study:
“…In healthy adults, C8 alone had the highest net ketogenic effect over 8 h, but induced only half the increase in the acetoacetate-to-β-HB ratio compared with CO. Optimizing the type of MCT may help in developing ketogenic supplements designed to counteract deteriorating brain glucose uptake associated with aging.…More
How Ketogenic Diets and Blueberries Make Your Brain Work Better
Dr. Robert Krikorian is Professor in the Department of Psychiatry & Behavioral Neuroscience and Director of the Cognitive Aging Program at the University of Cincinnati Academic Health Center.