Yes, but for ‘Leo Goldstein’?  Not his real name, I think, and - serious question here - why would anybody believe what ‘he’ says? It’s a mixture of deliberately deceptive stuff, cherry-picked things, and outright lies.
Not his real name, I think, and - serious question here - why would anybody believe what ‘he’ says? It’s a mixture of deliberately deceptive stuff, cherry-picked things, and outright lies.
“The use of Remdesivir for COVID-19 was authorized by the FDA based on a single RCT, conducted by NIAID with the participation of Gilead Sciences, the exclusive manufacturer of Remdesivir. A final report from this study was published on October 8, five months after the drug’s authorization.”
No, that’s a lie right there. Again, why in the world would anybody believe this, with only the claim from ‘Leo Goldstein’? It’s not true.
The change in October is full approval from the FDA for treatment of hospitalized patients 12 years old and older, and weighing 40 kg (88 lbs.) or more. There had been an Emergency Use Authorization in place for the drug (since May 1, I believe). The EUA has now been amended to allow for continued use, at this time, for hospitalized patients under 12 years old and/or weighing at least 3.5 kg (7.7 lbs.).
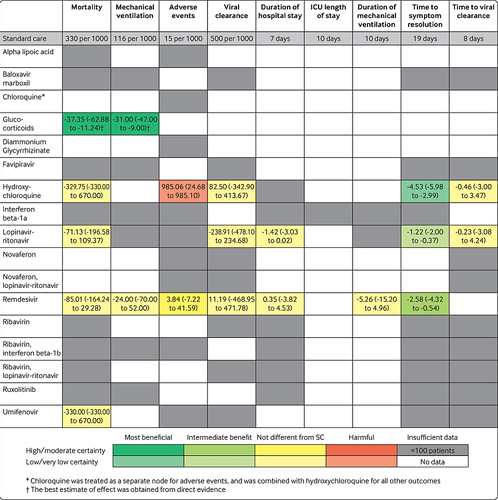
Nothing much has changed over the last couple months. When looking at all valid RCT studies, there still is no “miracle cure.” Nothing helps everybody, nor is there any guarantee for a given patient, ahead of time. Hydroxychloroquine does, on average, have a decent chance of making Covid-19 symptoms go away faster than with no drug at all. It’s not demonstrably better, in that respect, than the other drugs, however, and it does present more risk of harmful effects on patients.
In looking at peer-reviewed studies in the aggregate, hydroxychloroquine has improved in status - that red rectangle is now yellow, i.e. not substantially different from ‘standard care’ (none of the drugs in question being used). It remains much more likely to bring about adverse events for patients, however, versus Remdesivir, over 5 times more likely.

 Not his real name, I think, and - serious question here - why would anybody believe what ‘he’ says? It’s a mixture of deliberately deceptive stuff, cherry-picked things, and outright lies.
Not his real name, I think, and - serious question here - why would anybody believe what ‘he’ says? It’s a mixture of deliberately deceptive stuff, cherry-picked things, and outright lies.