I use both sucralose and erythritol. Both give me mild heartburn if I use it in large amounts but my goals are being met and it makes some things more enjoyable (like coffee, tea and yogurt). I’ve already given up the all day diet soda which took six months. It may be a very long time before I’m interested in taking all sweetness out of my life.
There is only one sweetener worth using!
If I am going to use anything, Erythitol is the closest to sugar. When I first started keto, I would have it sprinkled on blackberries with cream. Delicious! I have also made lemon possets with powdered erythritol for a dinner party that were enjoyed by everyone. I realised later that I hadn’t told anyone!
Nowadays I rarely use sweeteners and rarely eat fruit! I don’t think it overly affected me, I just gradually lost the desire.
I use non-GMO sugarcane alcohol (erythritol), as opposed to GMO corn slurry erythritol. I like the fact that erythritol isn’t metabolized by the body due to its molecular size - it just moves through and out. I buy maple flavored all-natural syrups from Amazon made out of erythritol mainly (Pyure brand, also Lakanto).
For making keto ice cream, I powder the erythritol in a coffee grinder, and it mixes fabulously. But for smoothies I add it straight to the blender and it disappears fine that way. To boost the sweetness, I add a couple drops of Stevia, which doesn’t have an aftertaste when accompanied by erythritol.
For certain dishes, I’ll use very small dabs of raw honey (1 tsp per person) to accompany winter brunch griddle cakes made w/ erythritol - or a 1 inch square of raw honeycomb in cream - as raw honey is an amazing paleo taste and carries so much life force, sunlight, and intelligence from the bees.
I do think if one is going to eat limited amounts of sweetened foods, one needs to counterbalance it in the eating window by also eating pungent, salty, bitter, sour - it keeps the palate well-rounded and thus the neurological stimulation.
I just don’t bother with any of them, don’t need that kind of sweetness in my life.
I just looked at the package and it has sugar and dextrose. Probably not a lot but why are those necessary at all?
They give it that brown glaze all over. At home we just cook it naturally and baste with fat - much more healthy!
Okay, in spite of the terrible name, I’m going to take yours and others word for it, and try some of that Erythritol…
Yes indeed, it’s a terrible name. I personally renamed it “Eartha” among my pals, cos I can’t stand having to pronounce 4 syllables and nonsense UNLESS it’s for a music band (ie, The Eurythmics).
The Anthony’s brand, online, is U.S. made, and the cheapest non-corn based I’ve found btw.
All this talk about artificial sugar sweeteners (makes my gag reflex go crazy just thinking about it) are a processed food/insecticide and that’s great if your an insect any way you slap it! If it tastes like chemicals that’s because it is!
Monk Fruit
Unsulfured (not extracted from bitter baby sugar cane) Light Molasses
Coconut Palm Sugar
Date Sugar
Raw Sugar Cane
Are the only ones I would use, if I consumed that kind of stuff which I do not. If it is not triggering my insulin or blood sugars then I would not eat it with the very rare exception of Monk Fruit.
Notes:
image link[1] “…The rate of glycolysis is also determined by the concentration of glucose, and the rate of gluconeogenesis by the concentrations of lactate and other precursors of glucose. …” …More
[2] Fatty acids CANNOT act as precursors for glucose?
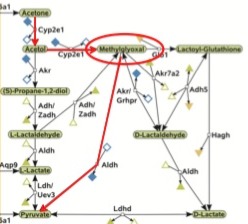
[3] We Really Can Make Glucose From Fatty Acids After All! O Textbook, How Thy Biochemistry Hast Deceived Me! “…Acetate would be converted to glucose through the TCA cycle and would thus not yield net synthesis of new glucose. I erased this pathway because we currently do not have evidence for acetone being converted to acetate without first being converted to pyruvate (Christoph Kaleta, senior author of reference 9, personal communication). In any case, the authors discussed evidence that this pathway is not active unless supraphysiological doses of acetone were given. In the latter case, they may have been observing conversion of pyruvate to acetate when the capacity for gluconeogenesis from pyruvate was saturated. …”
“…The authors of the computational analysis (9) calculated that the most cost-efficient way of converting fatty acids to glucose is by converting acetol to methylglyoxal, facilitated again by CYP2E1, and then converting methylglyoxal directly to pyruvate, facilitated by an enzyme called aldehyde dehydrogenase. I’ve outlined this pathway in red here:
As Peter Dobromylskyj over at Hyperlipid has pointed out before, methylglyoxal inhibits the breakdown of glucose. In a future post, I will cover methylgyloxal’s inhibition of glucose consumption in greater detail, but for now it is worth noting that when this pathway is activated, we not only convert fatty acids to glucose, but methylglyoxal concentrations rise and inhibit the breakdown of glucose. Thus, when glucose runs low and we begin subsisting primarily on fatty acids for fuel, we have a coordinated effort to both spare glucose and to make more of it. When the glucose recession hits, our cells do what any other budget-conscious cells would do and spend less. …”
[4] “…Aldehyde dehydrogenases are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes to carboxylic acids. The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. …” “…Acetaldehyde dehydrogenases (EC 1.2.1.10) are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetic acid. …More
[5] Aldehyde dehydrogenases (ALDHs): are a group of NAD[P] ± dependent enzymes that catalyze the oxidation of a wide variety of endogenous and exogenous aliphatic and aromatic aldehydes (Lindahl, 1992). …More …More
[7] Methylglyoxal pathway: The methylglyoxal pathway is an offshoot of glycolysis found in some prokaryotes, which converts glucose into methylglyoxal and then into pyruvate. Methylglyoxal is, however, a reactive aldehyde that is very toxic to cells, it can inhibit growth in E. coli at milimolar concentrations.
[8] RICK: Collaboration would be wonderful. And the other possibility is that the low carb diets improve insulin resistance in part because of reduction of dietary glucose that reduces endogenous fructose. When you become insulin resistant, your aldose reductase goes up and so you start making endogenous fructose. We’re doing studies right now with fructokinase knockout animals to specifically check that pathway.
STEVE (Phinney): I hadn’t considered endogenous fructose production .
RICK: It’s very important.
STEVE (Phinney): The pathways for disposal of dietary fructose are very different from the disposal in the fructose 6-phosphate pathway. They don’t cross. But endogenous fructose? Is there a place I can read about that. …More
[9] Sorbitol: less commonly known as glucitol, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group to a hydroxyl group. Most sorbitol is made from corn syrup, but it is also found in nature, for example in apples, pears, peaches, and prunes.[3] It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2.[4] While similar, the two sugar alcohols have very different sources in nature, melting points, and uses. …More
[10] “…Aldose reductase catalyzes the NADPH-dependent conversion of glucose to sorbitol, the first step in polyol pathway of glucose metabolism. The second and last step in the pathway is catalyzed by sorbitol dehydrogenase, which catalyzes the NAD-linked oxidation of sorbitol to fructose. Thus, the polyol pathway results in conversion of glucose to fructose with stoichiometric utilization of NADPH and production of NADH.[1]
The aldose reductase reaction, in particular the sorbitol produced, is important for the function of various organs in the body. For example, it is generally used as the first step in a synthesis of fructose from glucose; the second step is the oxidation of sorbitol to fructose catalyzed by sorbitol dehydrogenase. The main pathway from glucose to fructose (glycolysis) involves phosphorylation of glucose by hexokinase to form glucose 6-phosphate, followed by isomerization to fructose 6-phosphate and hydrolysis of the phosphate, but the sorbitol pathway is useful because it does not require the input of energy in the form of ATP:
- Seminal vesicles: Fructose produced from sorbitol is used by the sperm cells.
- Liver: Fructose produced from sorbitol can be used as an energy source for glycolysis and glyconeogenesis.
Aldose reductase is also present in the lens, retina, Schwann cells of peripheral nerves, placenta and red blood cells.[ citation needed] …” …More
[11] Mastering Nutrition Episode 6: Why “Glycation” Is a Bad Reason to Restrict Carbs
[14] What is the difference between dehydrogenase and reductase? A reductase is an enzyme that catalyzes a reduction reaction. Dehydrogenase are mainly responsible for oxidation of its substrates whereas reductase are mainly responsible for reduction of its substrates. “…A reductase is an enzyme that catalyzes a reduction reaction. …” …More
Ehhh…
For the most part.
But sucralose starts out as plain old table sugar, and is not changed a lot from its original form.
I’m more concerned about keeping my carbs down, and my fats up.
What happens way down the road is not worth worrying about too awfully much.
And I should have added, Splenda (sucralose) doesn’t taste like chemicals at all 

 your life is full of sweetness honey!
your life is full of sweetness honey!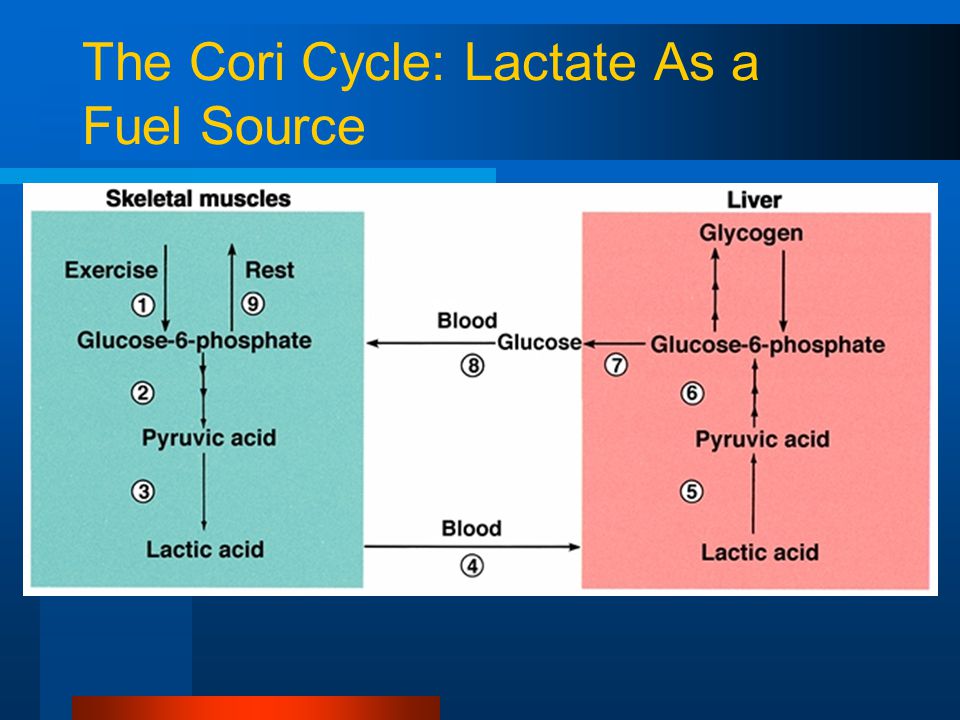

 Yeah, we all want stuff that ISN’T CHEMICALS.
Yeah, we all want stuff that ISN’T CHEMICALS.