I would say that ketones are the polar opposite of corrosive and toxic. They are beneficial signalling molecules of redox state and neuroprotective for a variety of things, including recovery from lack of oxygen due to stroke etc.
POLL! Plus an attempt so explain a few things about ketosis
One word: ketoacidosis. High octane mix of glucose and ketones. As I said, sufficient quantities over sufficient time…
Otherwise, yes, I agree ketones are great! Far better than long-term elevated glucose.
Yeah, we’re into pathology as opposed to nutritional ketosis at that point. I mean we’re basically talking about the difference between 0.5 and maybe 2.0 or 3.0 maximunm on a daily non-fasting basis. And if glucose goes down a bit because of the presence of ketones, so much the better. I’ve grown accustomed to not freaking out because my glucose isn’t in the 80’s all the time, which is what I used to take for ideal. Now, around 100 that stays constant all day is fine by me.
Fatty acids do not seem to be toxic or corrosive, unlike glucose. Fatty acid metabolism produces far fewer reactive oxygen species than glucose metabolism, nor do fatty acids cause advanced glycation end-products. Not only that, but the three ketone bodies all appear to act as hormones, as well as fuel. This is only to be expected from the proper human diet.
Context is important. In diabetic ketoacidosis, the problem is the absence of the regulatory effect of insulin on pH, which normally keeps the blood properly buffered, as witness the fact that people who ingest exogenous ketones can raise their serum β-hydroxybutyrate quite high without becoming acidotic. Whereas any quantity of serum glucose above the 4-5 mg required is problematic, as is the level of serum insulin required to deal with it.
If the dose makes the poison, the danger point for serum glucose and insulin is very close to the necessary minimum, whereas serum ketones have to increase by at least an order of magnitude to begin to be associated with any sort of problem. In that sense, glucose and insulin are like chlorine, damaging to the body in anything but trace amounts, whereas fat is like water, which is perfectly safe except in vast quantities that are hard to ingest.
Thanks! That is very interesting. With relatively normal insulin function then, FAs and ketones remain benign. That, of course, makes sense in an evolutionary sense. Presuming (I can’t cite anything) our primate ancestors and relatives were/are predominantly herbivores and glucose burners, humans gradually transitioned from glucose burning to fat burning. Sometime between 2-3 million years ago the transition was completed sufficiently that humans were predominantly fat burners. Just in time for the Pleistocene, fortunately, when deriving sustenance from plant sources became a losing proposition unless you still had the cellulose digesting gut of a herbivore. Ultimately, glucose burn remains as a remnant metabolic pathway still utilized but not optimal and easily disrupted.
As you note, kept within very narrow bounds, glucose remains useful. But all of a sudden and only a few moments ago, in the evolutionary sense, humans decided to domesticate plants and selectively breed them into ‘carbon sacks of sugar’. And here we are eye-witnessing the results of ignoring our evolution.
@PaulL I should also mention that ketones seem to be self-regulating. When enough unused ketones are floating around in the blood doing nothing much it looks like β-hydroxybutyrate reverts to acetoacetate and the acetoacetate ‘spontaneously’ disintegrates into acetone and CO2. As @carolT pointed out above, acetoacetate has a ‘half-life’. Most of the acetone exits the system very quickly either via the lungs or direct evaporation through tissue to the skin. As I recently pointed out, either above in this thread or in some other thread (can’t find right now, but will search) my BrAce readings decline with activity and increase with inactivity, which seems to support the above idea. It would be interesting if someone who tests their blood for β-hydroxybutyrate would do so hourly for a day or so to see what if any correlation they see between β-hydroxybutyrate levels and their activity through the day. Any takers…?
Ketones are also self-regulated by negative feedback through the “niacin” receptor on fat cells and through increased insulin varying with higher glucose levels.
http://www.jbc.org/content/280/29/26649.full
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1152056/ (rats)
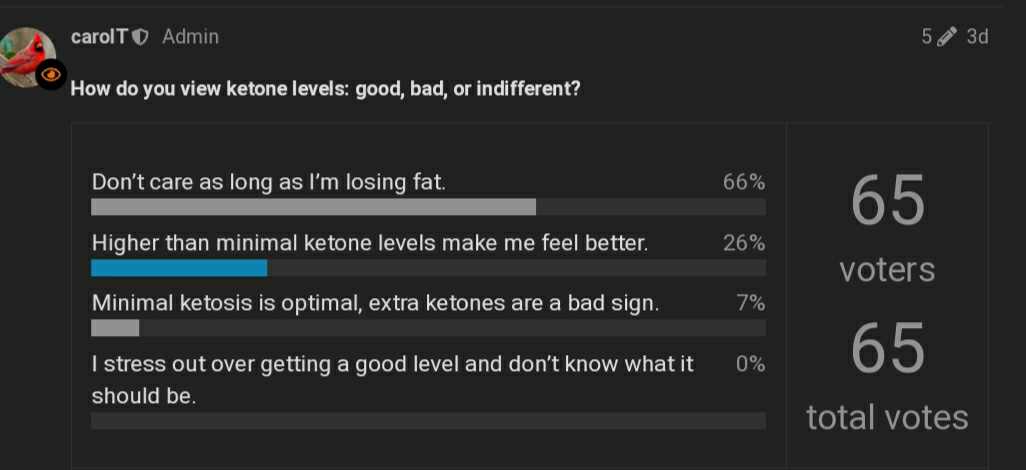
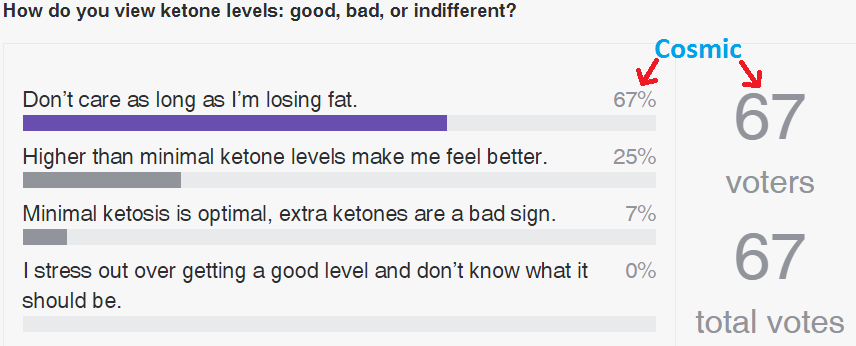
Results so far:
Thanks everyone! I have a better idea of the ratio of ketones are beneficial / don’t care people here. 
Poll will remain open indefinitely.
LOL. it wasn’t a contest, just a survey. Obviously, I’m in the minority, but Ithink its a significant minority. But then there’s clearly something wrong with my brain.

None of those answers worked for me.
I’m not doing this to lose weight.
I’m trying to stay in as deep a Therapeutic State of Ketosis as possible because I have Cancer.
It matters.
And I’m glad my Ketones are high.
~K-Bombs
Rabbit here, pokes head out of rabbit hole…lol
What regulates Somatostatin: DHA and EPA (from marine life) in addition to things like cold arctic environments or colder thermal conditions?
Ever wonder how fish keep warm in freezing water below zero? (when a chemical metabolism goes more into an electrical state)
References:
[1] “…The action of somatostatin 28 – inhibiting glucagon, inhibiting lipolysis, and sparing protein – is a useful backup to the similar effects of basal insulin. Basal insulin – the low background insulin level – supports a feedback loop by which ketones themselves inhibit lipolysis.[11] Together, this helps to explain why high fat diets (very low in carbohydrate, essentially ketogenic diets) WERE AS EFFECTIVE, and somewhat safer, than FASTING for reducing blood sugar and preventing diabetic ketoacidosis in the pre-insulin days, in people with low, but not zero, insulin production.[1] No hormone acts alone, and the regulatory mechanisms described above have been simplified. However, for people interested in high fat diets, somatostatin 28 is a potent hormone that specifically responds to dietary fat, and has actions which are desirable in a low-insulin context. …”…More
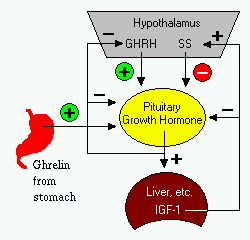
[2] Control of Growth Hormone Secretion: Production of growth hormone is modulated by many factors, including stress, exercise, nutrition, sleep and growth hormone itself. However, its primary controllers are two hypothalamic hormones and one hormone from the stomach (Ghrelin?):
•Growth hormone-releasing hormone (GHRH) is a hypothalamic peptide that stimulates both the synthesis and secretion of growth hormone. •Somatostatin (SS) is a peptide produced by several tissues in the body, including the hypothalamus. Somatostatin inhibits growth hormone release in response to GHRH and to other stimulatory factors such as low blood glucose concentration. •Ghrelin is a peptide hormone secreted from the stomach. Ghrelin binds to receptors on somatotrophs and potently stimulates secretion of growth hormone. Growth hormone secretion is also part of a negative feedback loop involving IGF-I. High blood levels of IGF-I lead to decreased secretion of growth hormone not only by directly suppressing the somatotroph, but by stimulating release of somatostatin from the hypothalamus. Growth hormone also feeds back to inhibit GHRH secretion and probably has a direct (autocrine) inhibitory effect on secretion from the somatotroph. Integration of all the factors that affect growth hormone synthesis and secretion lead to a pulsatile pattern of release. Basal concentrations of growth hormone in blood are very low. In children and young adults, the most intense period of growth hormone release is shortly after the onset of deep sleep. …” …More
[3] “…Previous studies have suggested that the C16 saturated fatty acid palmitate inhibits somatostatin secretion [40] but it is not known whether or not this reflects agonism at GPR120 since palmitate exerts pleiotropic effects in islet cells. Thus, we tested the effects of DHA, an n -3 fatty acid, which has been proposed as a physiological GPR120 agonist [2, 10, 11]. However, unlike Metabolex 36, DHA (up to 100 μmol/l) failed to attenuate glucose-induced somatostatin secretion. This may be because DHA exerts multiple effects on islet cells (including both metabolic and receptor-mediated responses) but, whatever the reason, these results emphasise the value of selective, synthetic agonists for delineating the role of GPR120 in islets. As confirmation that DHA was active under the conditions employed, insulin secretion was also studied and it was shown that the fatty acid dose-dependently potentiated insulin secretion from WT murine islets (Fig. 8b). However, it is interesting to note that DHA failed to increase insulin secretion from the Gpr120 -knockout islets. The reasons for this are unclear but the results could be taken to imply that activation of GPR120 plays a facilitatory role in mediating the enhanced insulin secretion. However, this conclusion is not supported by data obtained with the more selective GPR120 agonist Metabolex 36, which failed to promote insulin secretion under any condition studied (Figs 7a and and8b8b). …” …More
Oh no does that mean we are all contributing to GHG emissions like the cows?