My understanding is you eat fat and it’s hydrolysed into it’s constituent fatty acids so it can be absorbed. This is done by enzymes from the pancreas acting on the emulsified fats in bile.
Fatty acids with 14 or fewer carbons go straight into the blood leading to the liver. These are short and medium chained fatty acids. You can think of them as having an express pass straight to being burned in the liver. They get into your liver cells easily, and once in there they passively diffuse into the mitochondria where they are bet oxidized into energy units called Acetyl-CoA and fed into the Kreb cycle. We’ll get back to that process in a moment.
Fatty acids with longer than 14 carbons in their chains enter the cells of the gut (enterocytes) where they are re-esterified into triglycerides. The enterocytes build massive lipoprotein boats called Chylomicrons and they stuff them with triglycerides and any fat soluble vitamins from the gut. They then attach an addressing label to those boats (called an apoB-48) and they get shipped into the lymph to be taken up by veinous return and they go EVERWHERE except the liver. Every cell that is in the market for fat to use for energy puts out an ApoB receptor and when a Chylomicron docks the cell requests a little of it’s cargo and then the Chylomicron moves on. Fat cells will be taking up fat from the gut, so will the heart and other muscles.
Eventually the remnant of the chylomicron reaches the liver which expresses an ApoB receptor so it can drag it out of circulation. There will be some fat soluble vitamins, any triglycerides excess to cellular requirements, maybe a little cholesterol. The liver will use fat for energy if it can, it will also packages excess up into VLDL particles and give them a different address (ApoB-100) and send them off to any cell that expresses an ApoB receptor (and we’ve already met most of those).
Chylomicrons and LDLs aren’t the only way cells get fat to use for fuel. Fat cells, when insulin is low, release non-esterified fatty acids into sheets of a protein called albumin, and it travels the circulation being taken up by consumers of fat. Eventually they too make it back to the liver which cleans them out of circulation to use them for energy or ship them off again in LDL particles.
So now we have Medium and short chained fats in liver cells, and long chained ones from body fat or the diet. Short chained fats can diffuse into the mitochondria. Long chained have to get past a bouncer called teh Carnitine Shuttle (Carnitine Palmitoyl Transport or CPT). And when the cell is making fat, or when insulin is high, that shuttle is inhbited, and long chained fats have to take a different route.
They get cleaved into shorter chained fats in organelles called the peroxisomes in a process that produces a lot of hydrogen peroxide. Once they are shorter chained they can diffuse into the mitochondria, but the process is a lot slower than the CPT and it makes a lot of ROSs. [Sidebar this is why diabetics can’t burn their body fat and why their cells are so sick - they can’t get glucose into cells because they are insullin resistant, they can’t shuttle fat cos insulin is high, they have to use a polluting slow lane]
Finally fats in your mitochondria are cleaved into 2 carbon unit acetyls in a process called beta oxidation … and they are also present for the Kreb cycle to convert into energy.
I need a diagram to show the next bit from A particularly beautiful Infographic of metabolism
So normally 2 carbon acetyls get pumped into the kreb cycle from glucose or fat or glucogenic proteins, they meet a 4 carbon Oxaloacetate and become a 6 carbon citrate and go around the Kreb cycle which takes in oxygen and pulls off the 2 carbons making it into carbon dioxide and releasing a lot of energy.
So what if there isn’t an Oxaloacetate available?
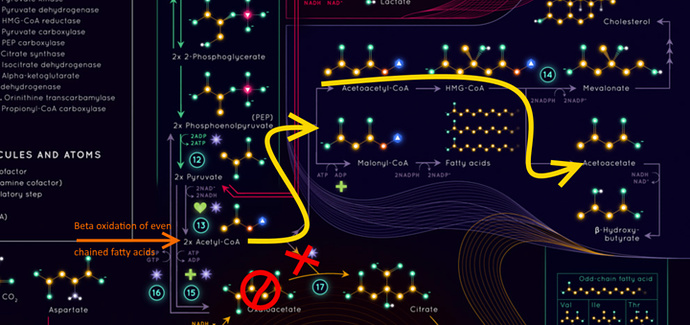
Well then when insulin is high then the Acetyl-CoA becomes a Malonyl-CoA which eventually triggers the creation of new fats (and inhibiting the carnitine shuttle from letting those newly created fats back into the club), or if insulin is low it becomes AcetoAcetatyl-CoA which we can make into a precursor to Cholesterol, or if we don’t need cholesterol we make it into AcetoiAcetate and eventually betahydroxybutyrate.
D-beta-hydroxybutyrate dehydrogenase is In the mitochondria.
The monocarboxylate transport is on the outside of a cell that lets specific molecules into the cell including lactate and pyruvate for making glucose, lactic acid from fermenting it, as well as the ketone bodies (BOHB, AcAc and Acetone) as well as some products of branched chain amino acids.
It is upregulated in the process of fat adaptation so we can take in AcAc spilling from liver cells and make it into shelf stable BOHB. Nice side benefit it helps us clear lactic acid from muscle cells working anaerobically.
Yup. When we get better at converting it into BOHB the levels drop.
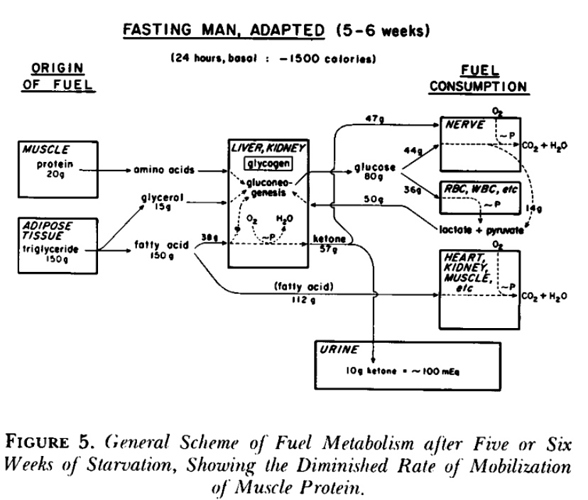
BOHB and AcAc and Acetone are all present in urine. Cahill determined a fat adapted man still urinates 10g a day but it’s a lot less AcAc once the fat adaptation has occured.