Just curious when using the ketostix…When it measures that you are burning fat…Is that fat from the food or is it fat that you are burning?
Just curious with Ketostix
As per the link that @OgreZed has provided: ketostix do NOT have anything to do with fat burn. The acetoacetate and possibly beta-hydroxybutyrate in urine are simply excess ketones dumped from the blood via the kidneys. Ketostix measures the ketones you are NOT burning. The only way to measure actual fat burn is to measure exhaled acetone, most easily in your breath with a breathalyzer. The only way to differentiate whether the fat you’re burning is from the plate or from your body is to measure/analyze your weight and fat loss over time. There are many options for doing that, some cheap/not very good, some expensive/better.
My advice is to not worry about testing, keeping your carbohydrates under 20g is guaranteed to put you in ketosis. Just trust the process, it’s what your body is designed to do and it will happen. After some weeks in ketosis you can back off on dietary fat and again you can be pretty sure you’re burning body fat because your clothes are getting looser and you look and feel different in a good way. That’s whether you’ve lost much weight or not. And getting hungry all the time isn’t an issue anymore.

There are some curious few (apparently) who like to test, measure, weigh, investigate, discover. We are not weirdos without a life. We just think pursuing our curiosity wherever it leads makes everything more interesting and satisfying. 
@amwassil I didn’t say anybody was a wierdo. It’s that there’s a certain waste of money involved in testing something when you’re not going to get accurate or relevant information from an investment that would be better spent on quality food. I know some people love their testing but it’s not really important in most people’s opinion and mine too. Just sharing my view. And I do like to do dietary tracking because I find it interesting. I use Cronometer. I can see that I am staying under 20 carbs and know I’m in ketosis all the time by how I feel.
@David_Stilley Fair enough and I meant no personal criticism of you. There are many on this forum who seem to think measuring and tracking anything is a waste of time/money better spent on grass-fed organic whatever. For a lot of us, though, measuring and tracking is important and helps us feel more in control and aware of what we’re doing. It also gives us the opportunity to discover things we didn’t know previously.
I don’t think ketostix are useless. If you detect any ketones in urine, you know that your liver is producing more than your cells and organs are using. That means you’re not burning fat as efficiently as you might. Of course, we can more effectively measure fat burn directly via exhaled acetone. Although, with the available consumer devices, there seems to be a wide range of reliability and accuracy. I’m hopeful that the technology will improve.
I think we might all agree that the most important thing is fat adaptation and fat burning. It doesn’t matter how many ketones the liver produces, what matters is how many get used to fuel the cells and organs. I think a lot of people misunderstand this and mistakenly think blood ketone readings tell them anything other than they’re in ketosis and their liver is synthesizing ketones. Blood ketones are available fuel, not necessarily used fuel. What does it matter if you measure 5.0mmol and you’re excreting half in your urine? I think it’s useful to know that.
As we all know, as fat adaptation improves and matures, ketone production tends to match energy requirements such that less and less gets excreted in urine. That’s useful to know and track. In my opinion.
Example. For unrelated reasons I started eating small amounts of raw ginger root a couple months ago. Shortly afterwards I purchased a cheap breathalyzer from Amazon after reading that the sensor used in the cheap ones doesn’t distinguish ethanol from acetone. Therefore, these cheap breathalyzers can be used to measure breath acetone (BrAce). There is a fairly long discussion about using these cheap devices on this forum, but I only discovered it after purchasing mine. The general consensus of that discussion was that these devices don’t tell you anything particularly useful. But I think they do.
BrAce is a direct measure of fat burn, since acetone is the breakdown product of burning fat and other ketones. I recognize that using a cheap breathalyzer designed to measure breath alcohol (BrAc) to test for acetone because of a sensor fluke is not likely to give me actual ppm acetone. This seems to have been the primary objection in the forum discussion. BUT, and this is an important BUT: as long as the numbers are consistent the device is in fact giving me useful information none-the-less.
Because it can be used easily and frequently (and requires NO blood letting!), the device can be used to record the trend of acetone production in my breath. That measurable trend in turn infers the trend in the underlying fat burn producing the acetone. I do not need to know actual ppm acetone to determine trends. If the device indicates that over a period of time my BrAce went up, I can logically conclude that fat burn increased; if down, that fat burn decreased; and, if steady, that fat burn remained about the same. I can also determine by how much; if one reading is .02% and the next reading is .04% I can conclude that ppm acetone more or less doubled, ie fat burn increased significantly.
Which leads me back to raw ginger root. I discovered that eating raw ginger root apparently increases fat burn significantly. If I ate 10-15 grams of raw root and went to bed an hour later with a breathalyzer display of .01%, upon awakening in the morning the breathalyzer might display .03% or .04% or .05%! In other words 3, 4 or 5 TIMES the concentration of acetone and by inference fat burn.
The increased fat burn was confirmed by an independent measure as well. During the 10 days that I informally tested raw ginger root as above, I lost 6+ pounds. In fact, I stopped the ‘experiment’ because I did not want to lose more weight. I do not attribute the weight loss exclusively to ginger root, because during that period of time my work schedule was such that I did not meet my daily calorie target most days. So I also had a 2-300 daily calorie deficit.
I find this very interesting and I think people trying to lose weight and/or stalling might find it very interesting as well.
Testing enhanced fat burn with raw whole ginger root
What the sticks and breath analyzers measure are wasted ketones, those that have been excreted without being used. So their primary value is to assure you that your liver is indeed producing ketone bodies. Whether they are manufactured from fat in your food or fat in your fat cells is impossible to determine, but if you are eating to satiety, your body is pegging your appetite to a level at which excess stored fat can be released from your fat cells for precisely this purpose. This assumes, of course, that you have excess stored fat to use; otherwise, your energy needs will come entirely from dietary fat.
The most accurate measure of ketogenesis is a blood meter, which measures the concentration of β-hydroxybutyrate in your bloodstream. Again, this is a measure of ketones you are not currently using, but it does give a reasonable indication of the rate at which your body is producing ketones. Unfortunately, there is no way to measure the total amount of fat being metabolised.
It is possible to calculate, from your muscle mass and your activity level, how much fat your muscles are burning (assuming you are now fat-adapted, of course), and it is theoretically possible to estimate roughly how much fat is being consumed to make ketones, but what you would end up with is a very rough estimate of fatty acid consumption. A better measure is your respiratory quotient, which measures the oxygen and carbon dioxide you breathe out. The ratio between them gives a fairly accurate indication of the degree to which the body is using glucose and fatty acids and in what percentages. I have no idea of how RQ is measured, but I doubt it can be done at home, alas!
That is not correct. Breath acetone (BrAce) is NOT wasted ketones, it is the byproduct of fat/ketone metabolism. The measurement of BrAce is directly related to fat burn and has been so documented in many studies, like these for example:
And, like these reports on research:
This is an interesting hypothesis:
As you point out:
“…which measures the concentration of of β-hydroxybutyrate in your bloodstream. Again, this is a measure of ketones you are not currently using…”
For whatever the measurement β-hydroxybutyrate in your bloodstream is useful, determining the efficiency of fat burn is not one of them. Measurement of BrAce is.
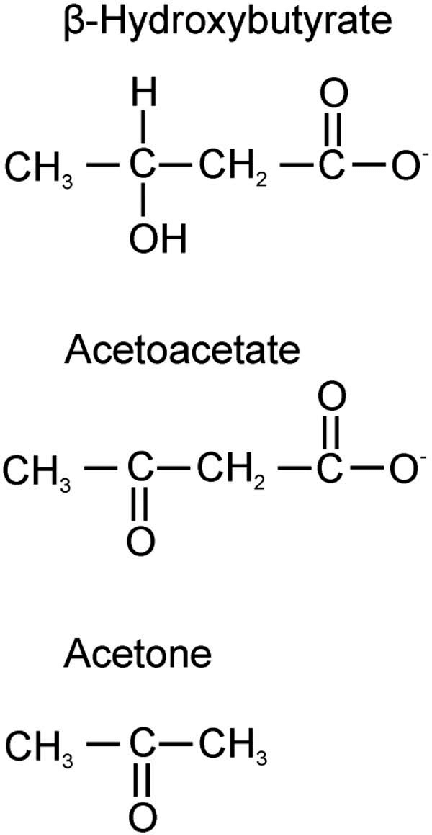
In simplified layman’s terms, it might be helpful to look at the following diagram. Both acetoacetate and β-hydroxybutyrate convert both ways into each other. The chemical differences between them are a loosely bonded hydroxide molecule (-OH) and a single hydrogen atom (-H). In acetoacetate the hydroxide molecule and the hydrogen atom in β-hydroxybutyrate are replaced by a single closely bonded oxygen atom (=O).
Please note that both acetoacetate and β-hydroxybutyrate contain a loosely bonded molecule composed of a single carbon atom (-C) and two oxygen atoms (O). That’s the bit on the right side of the diagram for both acetoacetate and β-hydroxybutyrate. That bit looks an awful lot like CO2!
Also note that acetone is exactly the same chemical structure as acetoacetate MINUS the carbon and oxygen (-CO2) on the right side of the diagram. The CH2 molecule of acetoacetate has been converted to CH3 because the CO2 molecule is gone, but the chemical properties are identical. Thus, acetone certainly looks like acetoacetate stripped of its CO2 molecule and released as the byproduct of metabolism along with the CO2. That transformation is accomplished during lipolysis, or what the rest of us call fat burn.
Yes, there is a small amount of acetone in the blood which can be measured as it makes it way to the lungs (mostly) and/or kidneys for excretion in either breath or urine. Since it is highly volatile, most of it exits via the lungs and through the skin rather than urine. Acetone is an end product of metabolism. It is technically a ketone body, but is not usable for energy and can not be converted back to acetoacetate. It is not a wasted ketone but a direct product of lipolysis and ketone metabolism and thus can be used to measure fat burn.
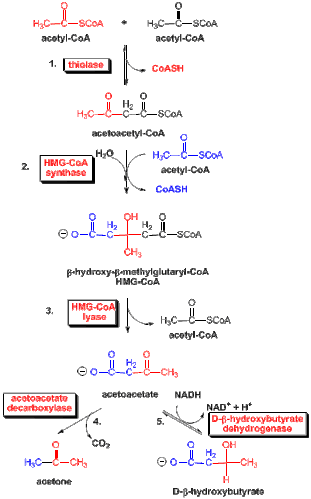
Here’s a diagram of lipolysis (fat metabolism). Note, at step3 both acetoacetate and acetyl-CoA are produced. The acetyl-CoA restarts the process again at step1. At step4 acetoacetate produces CO2 and acetone, as I noted in my previous comment. This is one way only: both CO2 and acetone are lost to the system (ie the ‘exhaust’ gases). At step5 acetoacetate and β-hydroxybutyrate transform to/from each other as fuel sources.
Acetone in the breath is by definition wasted, because it is being excreted, not metabolized for energy. Ketone bodies are intermediate products of fatty acid metabolism and still contain quite a bit of energy that the body could use. Ketones that are excreted cannot be used for energy, and that energy is therefore wasted as far as the body is concerned. Make sense?
Permit me to make a minor correction, because I fell afoul of it myself. The terms “lipolysis” and “fat metabolism” have distinct meanings. “Lipolysis” is defined as the breakdown of triglycerides into glycerol and their component fatty acids, whereas “fat metabolism” is the rest of the process of turning those fatty acids into adenosine triphosphate, water, and carbon dioxide. Personally, I think the term “lipolysis” ought to have the latter definition, but they did not consult me, alas!
By the way, the diagram you show is only the first half of the process of fat metabolism, the rest of it involving running the ketone bodies shown in the diagram through the citric acid cycle, etc.
Do you also think that CO2 is by definition ‘wasted’ because it is not metabolized for energy? Acetone and CO2 are the end products of acetoacetate metabolism and can not be used for energy. They are the final and irreversible results of the utilization of an acetoacetate molecule as fuel. In other words, a molecule of acetoacetate has ‘burned’ to produce metabolic energy and the ‘exhaust’ of that ‘burn’ are a molecule of CO2 and a molecule of acetone. Nothing is ‘wasted’. Both are molecules of no use and are dumped from the system. Perhaps you think because acetone is a ketone body it therefore must be available for energy. Not so.
I appreciate that. I’ve skipped over lots of things in my attempt at simplification of a VERY complex synthesis of interrelated processes. 
Not only because the process is confusing, but because I want to focus on the oxidation (what I’m calling ‘burn’) of acetoacetate. That is an end process, when a molecule of acetoacetate actually transfers its contained energy to some cell or organ and disappears in a puff of CO2 and acetone. Because of their volatility, both CO2 and (especially) acetone make their way quickly via the bloodstream to the lungs for exhalation. In fact acetone is so volatile it is not contained by the bloodstream and some of it actually evaporates directly through the skin!
A further complication is that aceteoacetate can ‘spontaneously’ immolate itself in a puff of CO2 and acetone as it simply floats around in the bloodstream waiting to be used for energy by a cell somewhere. That little spontaneous burst of molecular energy is as close to ‘wasted’ as it’s going to get but even that little burst warms the blood a little if nothing else.
My guess is that the so-called ‘spontaneous’ breakdown of acetoacetate results from a positive energy imbalance of too many ketones in the bloodstream. Like insulin packing excess glucose into the cells, acetoacetate immolates in a little puff of heat.
This is interesting:
Yes, indeed. It is a waste product of fat metabolism, among other things.
Not quite right. CO2 is not metatabolizable, but acetone, one of the ketone bodies, is an intermediate product of fat metabolism and can be metabolized further to ATP + CO2+ H2O. Generating the ATP is the point, of course, since it is the use of ATP that generates energy for the cell at large.
Exhaled acetone is not ‘wasted’ in the same sense that acetoacetate flushed out in urine is ‘wasted ketones’. The acetoacetate molecule IS the energy package. Upon delivering the energy to a cell the molecule of acetoacetate ceases to exist and a molecule of CO2 and a molecule of acetone remain. These are flushed out of the cell as non-usable, toxic ‘waste’, not unused energy. Because some of the acetone might be recaptured, detoxified and recycled as a part of metabolically active molecules, does not mean that the the bulk of it getting exhaled or evaporated through the skin is ‘wasted ketones’. Acetone requires both capture and conversion, ie energy input.
I would be very surprised if a couple million years down the evolutionary path our metabolism is not utilizing as much of that acetone as it can for energy. Acetone IS a toxic chemical and requires detoxification before any of it can be recycled (via CYP2E1). My guess is that its toxicity and volatility are the limiting factors. The energy required to capture and detoxify it is more than the energy return it has to offer. In other words there are easier ways to get carbon, oxygen and hydrogen into the system.
Some small percentage of acetone from acetoacetate burn ends up evaporating out of the bloodstream and out of the system through the skin. Some percentage (to be determined) is recaptured and detoxified for eventual conversion back to Acetal-CoA to glucose and/or via ATP as you indicate. Simply because all acetone is not recaptured/detoxified for conversion into some usable form, exhaled and evaporated acetone are not ‘wasted ketones’. I agree with you that they represent some amount of potential energy lost to the system, but I suspect that occurs because the energy cost of tapping that potential probably exceeds the potential energy gain.
Even though some acetone can be ‘captured’, treated as a toxic xenobiotic and detoxified for additional metabolic use, acetone remains an end product, like CO2, of the liberation of energy from acetoacetate, as the above chemical diagrams I’ve cited show clearly. Maybe the chemical diagrams and equations need to be revisited.
http://www.bioline.org.br/request?np10002
https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1965.tb47455.x
https://www.sciencedirect.com/science/article/pii/0041008X72900324?via%3Dihub
Journal of Biological Chemistry 1987-Gavino-6735-40-2 PDF
https://www.ncbi.nlm.nih.gov/pubmed/7778975
None of this invalidates measuring BrAce as a direct determinant of fat burn. Exhaled acetone is in fact a very good measure acetoacetate burn.
Oh boy! I wish I was clever enough to follow you guys! Each time one of you posts, I say to myself: yeah, that sounds right! When you reach consensus (hmmmmm…), can you pls let me know, my head is spinning.