[quote=“ron-coleman, post:431, topic:8415, full:true”]
I believe that @richard has mentioned numerous times that protein takes more internal energy to burn which is good and bad.[/quote]
You’ve probably heard about the Atwater factors 4/4/9 - where protein and carbohydrates produce 4 kCal/g, and fats produce 9 kCal/g but that’s not entirely correct.
The reality is energy is all about the number of hydrogen bonds and there is not a linear relationship between those and molecular weight of the various sources of energy. The heat of combustion of glucose is 3.95 and starch as high as 4.2.
Fats differ depending on the fatty acids contained, from 9.25 for Dairy fats to 9.5 for meat.
The energy of combustion of protein is not quite 8, but it is a lot more than 4. For vegetable protein it’s 5.0 and 5.8 for grain protein, and averages around 5.65 kCal. But we lose some of proteins potential energy before we can convert it into energy in our mitochondria which comes down to how amino acids are made .
Source: Exercise Physiology: Nutrition, Energy, and Human Performance
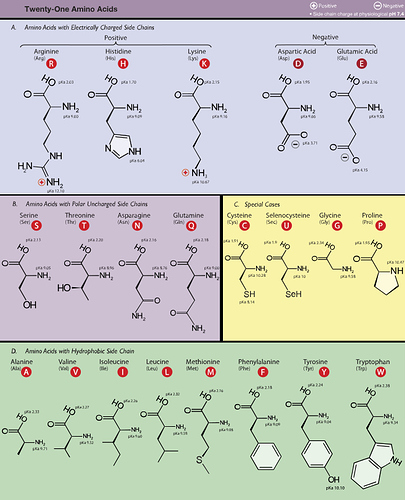
Protein is made from long chains of amino acids all linked together. Generally speaking amino acids are molecules with a carbon spine sporting legs made of hydrogen and oxygen atoms just like fats and glucose, but they have extra units that have to be removed first before they can be burned for fuel. The most common “extra bit” is an NH2 sometimes called an amine group (which is how amino acids get part of their name). Rare ones also have Sulphur and Selenium and an aromatic ring but lets focus on the amines.
If you burn a protein completely in a BOM calorimeter you’ll get roughly 5.65 kCal/g but we’re not combustion chambers - we have to turn them into acetyl-CoA so we need to first strip off the Nitrogens. Each Amine unit we remove steals an additional hydrogen in order to turn it into an Ammonia molecule (NH3) and each of those lost hydrogen bonds is wasted energy. If you look at that family portrait of amino acids some have 1 amine, some have 2, some have 3, and arginine has 4. The more nitrogen atoms the more energy will be stolen just to make them into fuel.
So it depends on context. On average it’s roughly 19% of energy giving an average of 4.6 kCal/g to the body in ATP energy but depending on which amino acids are used from 3.11 kCal/g for vegetables to 4.37 kCal/g for eggs.
Protein is an awesome lego block to make humans from … it’s a lousy fuel.
But it doesn’t really matter how many calories these produce, even if we could measure them accurately. That’s for our body to worry about … all we have to worry about is listening to its hunger and satiation cues.