The activation of the generation of ketones is said to start at the point when liver glycogen is depleted. For someone burning primarily glucose this seems to be fairly easy to calculate- it takes around 24 hours I believe if one were to fast or it could sped up with the help of glycolic exercise. But for those that have been fat adapted for a while, are lean and physically active, is liver glycogen ever completely depleted, either by virtue of fasting or through high intensity exercise? And if so, how is substrate utilized to fill those stores up?
Is liver glycogen ever fully depleted/topped off
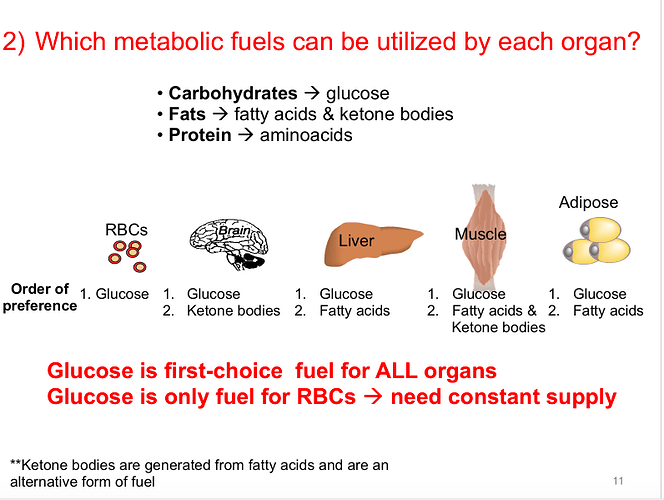
I don’t believe our bodies will allow us to fully deplete our glycogen since there are some cells in our body that require glucose. These are called obligate glucose users and red blood cells are one example. In the absence of carbs, our bodies can produce glucose through gluconeogenesis and this not only feeds OGUs it helps us keep our blood glucose levels stable. Without this adaptation we would be at risk for hypoglycemia.
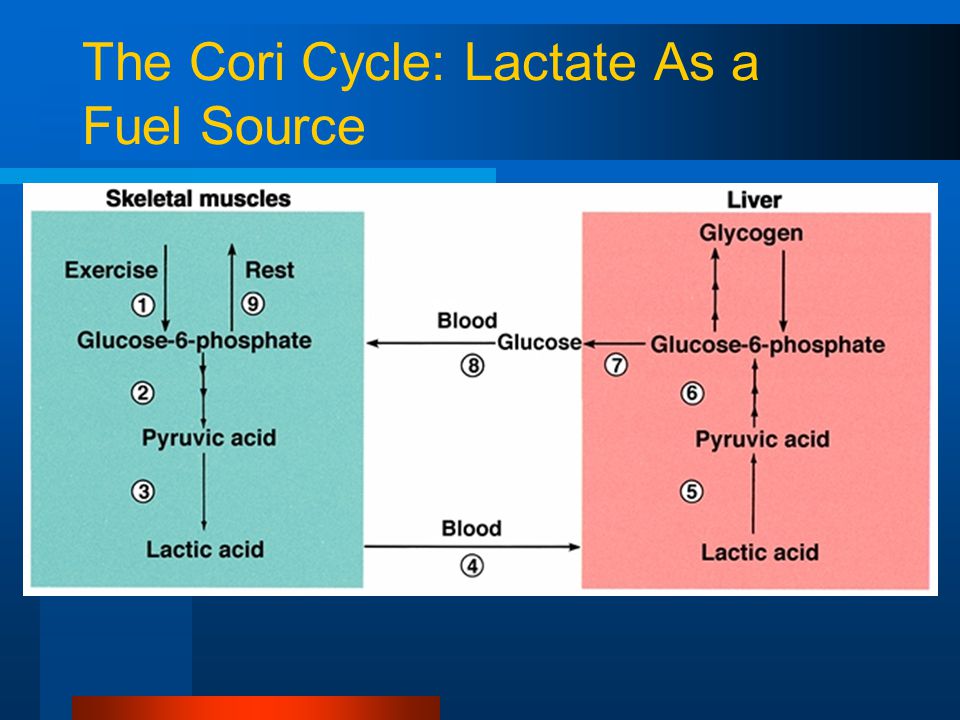
In regards to physical activity depleting our stored glycogen, we can produce glucose from the lactate our muscles produce when in an aerobic state, through a process called the Cori Cycle… which I find pretty amazing. The problem with all that lactate is the residual burn it causes in our muscles.
https://www.breaknutrition.com/what-is-gluconeogenesis/
https://peterattiamd.com/the-interplay-of-exercise-and-ketosis-part-i/
I like Peter Attia, and he talks a lot about lactate threshold, which I find fascinating as a personal trainer. I wish there were more info on there about liver glycogen depletion while eating LCHF. I suppose what I’m wondering about is if someone is very well fat adapted is it necessary to deplete liver glycogen to start producing ketones? There was an info graphic Ted Naiman just posted that demonstrated the trajectory from burning glucose to burning ketones, but it’s short on extrapolating on different scenarios…
The way I understand it is that when we first start LCHF, our glycogen reserves in our liver and muscles are burned and that accounts for much of the initial water weight we lose. When we do eat a small amount of carbs along with LCHF, our body prioritizes the carbs for energy use, which is why our ketone levels drop after eating. I don’t believe carbs eaten in small amounts get stored as long as the amount is not significant. But if we go hog wild on a cheat meal, people tend to gain five or more pounds overnight and I believe this is the excess glycogen being stored in our liver and muscles.
In this scenario, GNG is our source of glucose but I’m not 100% sure if anything is stored for a rainy day. From what I understand, GNG is driven by demand, not supply.
Maybe @paull can shed some light on your question better.
Some nice infographics for visuals:
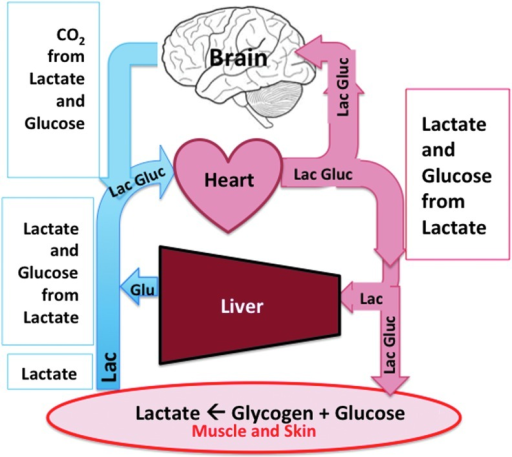
RBC’s Cellular processes: As a result of not containing mitochondria, red blood cells use none of the oxygen they transport; instead they produce the energy carrier ATP by the glycolysis of glucose and lactic acid fermentation on the resulting pyruvate. …More
That last sentence really gets me?
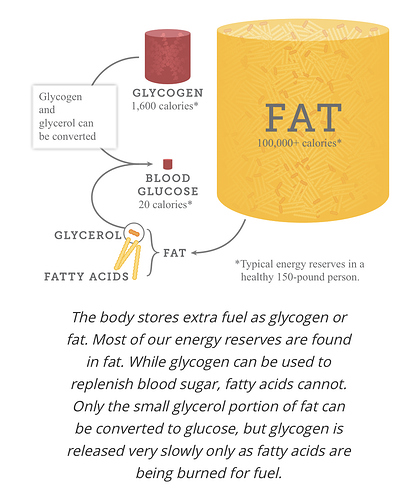
“…Only the small glycerol portion of fat can be converted to glucose, but glycogen is released very slowly as fatty acids are being burned for fuel? …”
#81 Bec Johnson
Not entirely sure, but liver glycogen can be shared (possibly be being converted back to glucose, I don’t know), whereas once the muscle makes glycogen, it has to stay in the muscle and be metabolized. I’d guess that the liver maintains a small reserve of glycogen as a cushion, but that’s purely a guess.
This even gets more perplexing:
“…New Study to Determine the Role of Glucose & Fructose in Disease: (very long you will have to scroll down to it, to get to the excerpted dialogue I am citing)
Dialog:
RICK: Collaboration would be wonderful. And the other possibility is that the low carb diets improve insulin resistance in part because of reduction of dietary glucose that reduces endogenous fructose. When you become insulin resistant, your aldose reductase goes up and so you start making endogenous fructose. We’re doing studies right now with fructokinase knockout animals to specifically check that pathway.
STEVE (Phinney): I hadn’t considered endogenous fructose production.
RICK: It’s very important.
STEVE (Phinney): The pathways for disposal of dietary fructose are very different from the disposal in the fructose 6-phosphate pathway. They don’t cross. But endogenous fructose? Is there a place I can read about that. …More
Notes:
[2] Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification “…Fructose concentrations in blood and tissues Fructose concentrations in blood
The normal systemic blood concentration in humans is in the low μM range when no fructose is being absorbed [68], and up to the low mM range after eating, depending on the fructose concentration in the diet [43]. Several studies have reported comparable serum and urinary fructose concentrations in diabetic patients and non-diabetic patients [69,70], while other investigators found increased levels in diabetic patients [68]. This discrepancy might be explained by an insufficient removal of glucose by the methods used, which interfered with the measurement of small amounts of fructose, or by an impaired precision of the measurements by complicated pre-treatment. In a recently described method, these problems were overcome by the use of 13C6-fructose as internal standard [68]. Serum fructose concentrations in patients with diabetes (12 ± 4 μmol/L) were significantly higher than those in healthy subjects (8 ± 1 μmol/L) and were correlated significantly with HbA1c. In patients with diabetes, the serum fructose concentrations decreased rapidly, concomitantly with an improvement in glycaemia [68]. Therefore, hyperglycaemia is associated with increased serum and urinary fructose concentrations in patients with diabetes. …”
[6] “…Due to increased blood glucose levels, methylglyoxal has higher concentrations in diabetics and has been linked to arterialatherogenesis. Damage by methylglyoxal to low-density lipoprotein through glycation causes a fourfold increase of atherogenesis in diabetics.[11] Although methylglyoxal has been shown to increase carboxymethyllysine levels,[12] methylglyoxal has been suggested to be a better marker for investigating the association between AGEs with adverse health outcomes. Methylglyoxal is a component of some kinds of honey, including manuka honey; it appears to have activity against E. coli and S. aureus and may help prevent formation of biofilmsformed by P. aeruginosa .[13] …” …More
[7] Methylglyoxal—A Potential Risk Factor of Manuka Honey in Healing of Diabetic Ulcers
Note: Methylglyoxal levels are the same in high carb or low carb high fat dieters, no difference?
You always have such wonderful resources @atomicspacebunny…but I may be more confused now after reading through some of those descriptions 
All interconnected factors but back to your original question “…is liver glycogen ever depleted/topped off?..”
Yes! They call it a depletion work out!
See also: Glycogen Depletion: Signs, Symptoms and Working Out